Dipalmitoylation of radicicol results in improved efficacy against tumor growth and angiogenesis in vivo
- PMID: 17233839
- PMCID: PMC11158274
- DOI: 10.1111/j.1349-7006.2006.00359.x
Dipalmitoylation of radicicol results in improved efficacy against tumor growth and angiogenesis in vivo
Abstract
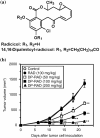
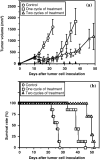
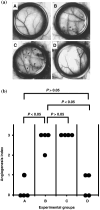
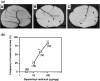
Tumor-related angiogenesis is likely to be a potential target for the treatment of cancer. One key to develop this angiostatic strategy would be to find useful angiogenesis inhibitors. Here we report the effects of radicicol, a microbial angiogenesis inhibitor that we previously identified using the chorioallantoic membrane assay, and its novel analog, 14,16-dipalmitoyl-radicicol, on tumor angiogenesis and growth. As expected for agents containing a penolic hydroxyl group, systemic administration of radicicol had little or no effect on neovascularization triggered by a M5076 mouse tumor cell line or a RMT-1 rat mammary carcinoma cell line established from autochthonous rat mammary tumors induced by 7,12-dimethylbenz[a]anthracene in a mouse dorsal air sac assay system. The agent did not show growth-inhibitory activity against either transplantable M5076 tumors or autochthonous 7,12-dimethylbenz[a]anthracene-induced rat mammary tumors. In contrast, 14,16-dipalmitoyl-radicicol potently suppressed tumor angiogenesis and growth in these experimental models. Furthermore, the analog significantly prolonged the survival rate of M5076-implanted mice. Although not stronger than radicicol, it dose-dependently inhibited embryonic angiogenesis in the chorioallantoic membrane assay, the dose required for half-maximal inhibition (ID(50)) value being 23 microg (27 nmol) per egg, and showed concentration-dependent antiproliferative activity against microvascular endothelial cells in vitro. These data suggest that 14,16-dipalmitoyl-radicicol is a promising antitumor agent with antiangiogenic activity.
Figures


Similar articles
-
PE, a new sulfated saponin from sea cucumber, exhibits anti-angiogenic and anti-tumor activities in vitro and in vivo.Cancer Biol Ther. 2005 Aug;4(8):874-82. doi: 10.4161/cbt.4.8.1917. Cancer Biol Ther. 2005. PMID: 16082187
-
Glipizide, an antidiabetic drug, suppresses tumor growth and metastasis by inhibiting angiogenesis.Oncotarget. 2014 Oct 30;5(20):9966-79. doi: 10.18632/oncotarget.2483. Oncotarget. 2014. PMID: 25294818 Free PMC article.
-
Inhibition of tumor angiogenesis by targeting endothelial surface ATP synthase with sangivamycin.Jpn J Clin Oncol. 2007 Nov;37(11):867-73. doi: 10.1093/jjco/hym115. Epub 2007 Oct 23. Jpn J Clin Oncol. 2007. PMID: 17956898
-
Inhibition of angiogenesis by rhizoxin, a microbial metabolite containing two epoxide groups.Jpn J Cancer Res. 1997 Dec;88(12):1125-9. doi: 10.1111/j.1349-7006.1997.tb00339.x. Jpn J Cancer Res. 1997. PMID: 9473728 Free PMC article.
-
The chick embryo chorioallantoic membrane in the study of tumor angiogenesis.Rom J Morphol Embryol. 2008;49(2):131-5. Rom J Morphol Embryol. 2008. PMID: 18516317 Review.
Cited by
-
Interaction of cepharanthine with immobilized heat shock protein 90α (Hsp90α) and screening of Hsp90α inhibitors.Anal Biochem. 2013 Mar 1;434(1):202-6. doi: 10.1016/j.ab.2012.11.010. Epub 2012 Dec 3. Anal Biochem. 2013. PMID: 23219559 Free PMC article.
-
Reduced Contractility and Motility of Prostatic Cancer-Associated Fibroblasts after Inhibition of Heat Shock Protein 90.Cancers (Basel). 2016 Aug 24;8(9):77. doi: 10.3390/cancers8090077. Cancers (Basel). 2016. PMID: 27563925 Free PMC article.
-
Nobiletin, a citrus polymethoxyflavonoid, suppresses multiple angiogenesis-related endothelial cell functions and angiogenesis in vivo.Cancer Sci. 2010 Nov;101(11):2462-9. doi: 10.1111/j.1349-7006.2010.01668.x. Cancer Sci. 2010. PMID: 20670297 Free PMC article.
References
-
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995; 1: 27–31. - PubMed
-
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996; 86: 353–64. - PubMed
-
- Risau W. Mechanism of angiogenesis. Nature 1997; 386: 671–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

