Metabolic mapping by use of high-resolution magic angle spinning 1H MR spectroscopy for assessment of apoptosis in cervical carcinomas
- PMID: 17233882
- PMCID: PMC1781459
- DOI: 10.1186/1471-2407-7-11
Metabolic mapping by use of high-resolution magic angle spinning 1H MR spectroscopy for assessment of apoptosis in cervical carcinomas
Abstract
Background: High-resolution magic angle proton magnetic resonance spectroscopy (HR 1H MAS MRS) provides a broad metabolic mapping of intact tumor samples and allows for microscopy investigations of the samples after spectra acquisition. Experimental studies have suggested that the method can be used for detection of apoptosis, but this has not been investigated in a clinical setting so far. We have explored this hypothesis in cervical cancers by searching for metabolites associated with apoptosis that were not influenced by other histopathological parameters like tumor load and tumor cell density.
Methods: Biopsies (n = 44) taken before and during radiotherapy in 23 patients were subjected to HR MAS MRS. A standard pulse-acquire spectrum provided information about lipids, and a spin-echo spectrum enabled detection of non-lipid metabolites in the lipid region of the spectra. Apoptotic cell density, tumor cell fraction, and tumor cell density were determined by histopathological analysis after spectra acquisition.
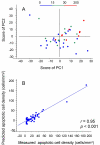
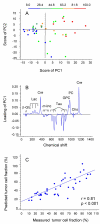
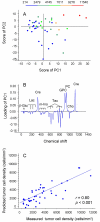
Results: The apoptotic cell density correlated with the standard pulse-acquire spectra (p < 0.001), but not with the spin-echo spectra, showing that the lipid metabolites were most important. The combined information of all lipids contributed to the correlation, with a major contribution from the ratio of fatty acid -CH2 to CH3 (p = 0.02). In contrast, the spin-echo spectra contained the main information on tumor cell fraction and tumor cell density (p < 0.001), for which cholines, creatine, taurine, glucose, and lactate were most important. Significant correlations were found between tumor cell fraction and glucose concentration (p = 0.001) and between tumor cell density and glycerophosphocholine (GPC) concentration (p = 0.024) and ratio of GPC to choline (p < 0.001).
Conclusion: Our findings indicate that the apoptotic activity of cervical cancers can be assessed from the lipid metabolites in HR MAS MR spectra and that the HR MAS data may reveal novel information on the metabolic changes characteristic of apoptosis. These changes differed from those associated with tumor load and tumor cell density, suggesting an application of the method to explore the role of apoptosis in the course of the disease.
Figures


References
-
- Hockel M, Schlenger K, Hockel S, Vaupel P. Hypoxic cervical cancers with low apoptotic index are highly aggressive. Cancer Res. 1999;59:4525–4528. - PubMed
-
- Imoto I, Tsuda H, Hirasawa A, Miura M, Sakamoto M, Hirohashi S, Inazawa J. Expression of cIAP1, a target for 11q22 amplification, correlates with resistance of cervical cancers to radiotherapy. Cancer Res. 2002;62:4860–4866. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

