A long-term "memory" of HIF induction in response to chronic mild decreased oxygen after oxygen normalization
- PMID: 17233898
- PMCID: PMC1783864
- DOI: 10.1186/1471-2261-7-4
A long-term "memory" of HIF induction in response to chronic mild decreased oxygen after oxygen normalization
Abstract
Background: Endothelial dysfunction (ED) is functionally characterized by decreased vasorelaxation, increased thrombosis, increased inflammation, and altered angiogenic potential, has been intimately associated with the progression and severity of cardiovascular disease. Patients with compromised cardiac function oftentimes have a state of chronic mild decreased oxygen at the level of the vasculature and organs, which has been shown to exacerbate ED. Hypoxia inducible factor (HIF) is a transcription factor complex shown to be the master regulator of the cellular response to decreased oxygen levels and many HIF target genes have been shown to be associated with ED.
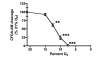
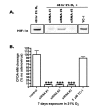
Methods: Human endothelial and aortic smooth muscle cells were exposed either to A) normoxia (21% O2) for three weeks, or to B) mild decreased oxygen (15% O2) for three weeks to mimic blood oxygen levels in patients with heart failure, or to C) mild decreased oxygen for two weeks followed by one week of normoxia ("memory" treatment). Levels of HIF signaling genes (HIF-1alpha, HIF-2alpha, VEGF, BNIP3, GLUT-1, PAI-1 and iNOS) were measured both at the protein and mRNA levels.
Results: It was found that chronic exposure to mild decreased oxygen resulted in significantly increased HIF signaling. There was also a "memory" of HIF-1alpha and HIF target gene induction when oxygen levels were normalized for one week, and this "memory" could be interrupted by adding a small molecule HIF inhibitor to the last week of normalized oxygen. Finally, levels of ubiquitylated HIF-1alpha were reduced in response to chronic mild decreased oxygen and were not full restored after oxygen normalization.
Conclusion: These data suggest that HIF signaling may be contributing to the pathogenesis of endothelial dysfunction and that normalization of oxygen levels may not be enough to reduce vascular stress.
Figures



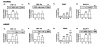


References
-
- Landmesser U, Hornig B, Drexler H. Endothelial function: a critical determinant in atherosclerosis? Circulation. 2004;109:II27–33. - PubMed
-
- Aronson D, Rayfield EJ, Chesebro JH. Mechanisms determining course and outcome of diabetic patients who have had acute myocardial infarction. Ann Intern Med. 1997;126:296–306. - PubMed
-
- Britland ST, Young RJ, Sharma AK, Clarke BF. Relationship of endoneurial capillary abnormalities to type and severity of diabetic polyneuropathy. Diabetes. 1990;39:909–913. - PubMed
-
- Farhangkhoee H, Khan ZA, Kaur H, Xin X, Chen S, Chakrabarti S. Vascular endothelial dysfunction in diabetic cardiomyopathy: Pathogenesis and potential treatment targets. Pharmacol Ther. 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

