The crystal structure of C2a, the catalytic fragment of classical pathway C3 and C5 convertase of human complement
- PMID: 17234210
- PMCID: PMC1868468
- DOI: 10.1016/j.jmb.2006.12.039
The crystal structure of C2a, the catalytic fragment of classical pathway C3 and C5 convertase of human complement
Abstract
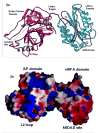
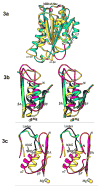
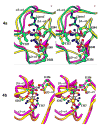
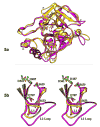
The multi-domain serine protease C2 provides the catalytic activity for the C3 and C5- convertases of the classical and lectin pathways of complement activation. Formation of these convertases requires the Mg(2+)-dependent binding of C2 to C4b, and the subsequent cleavage of C2 by C1s or MASP2, respectively. The C-terminal fragment C2a consisting of a serine protease (SP) and a von Willebrand factor type A (vWFA) domain, remains attached to C4b, forming the C3 convertase, C4b2a. Here, we present the crystal structure of Mg(2+)-bound C2a to 1.9 A resolution in comparison to its homolog Bb, the catalytic subunit of the alternative pathway C3 convertase, C3bBb. Although the overall domain arrangement of C2a is similar to Bb, there are certain structural differences. Unexpectedly, the conformation of the metal ion-dependent adhesion site and the position of the alpha7 helix of the vWFA domain indicate a co-factor-bound or open conformation. The active site of the SP domain is in a zymogen-like inactive conformation. On the basis of these structural features, we suggest a model for the initial steps of C3 convertase assembly.
Figures
References
-
- Volanakis JE. Overview o the Complement System. In: Volanakis JE, Frank MM, editors. The Juman Complement System. Marcel Dekker, Inc; New York: 1998.
-
- Kamata T, Liddington RC, Takada Y. Interaction between collagen and the alpha(2) I-domain of integrin alpha(2)beta(1). Critical role of conserved residues in the metal ion-dependent adhesion site (MIDAS) region. Journal of Biological Chemistry. 1999;274:32108–11. - PubMed
-
- Arlaud GJ, Volanakis JE, Thielens NM, Narayana SV, Rossi V, Xu Y. The atypical serine proteases of the complement system. Adv Immunol. 1998;69:249–307. - PubMed
-
- Horiuchi T, Macon KJ, Kidd VJ, Volanakis JE. cDNA cloning and expression of human complement component C2. J Immunol. 1989;142:2105–11. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

