Azetidinones as vasopressin V1a antagonists
- PMID: 17234419
- PMCID: PMC2067992
- DOI: 10.1016/j.bmc.2006.12.031
Azetidinones as vasopressin V1a antagonists
Abstract
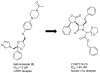
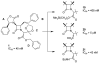
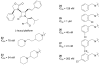
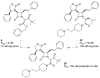
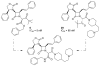
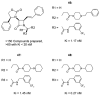
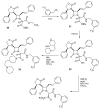
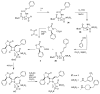
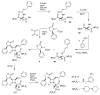
The azetidinone LY307174 (1) was identified as a screening lead for the vasopressin V1a receptor (IC50 45 nM at the human V1a receptor) based on molecular similarity to ketoconazole (2), a known antagonist of the luteinizing hormone releasing hormone receptor. Structure-activity relationships for the series were explored to optimize receptor affinity and pharmacokinetic properties, resulting in compounds with Ki values <1nM and brain levels after oral dosing approximately 100-fold higher than receptor affinities.
Figures

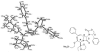
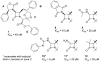
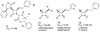
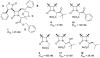
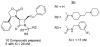
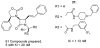

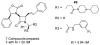
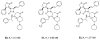

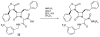
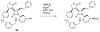
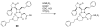
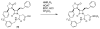
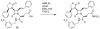
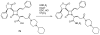
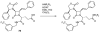

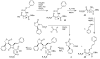
References
-
- Jard S, Elands J, Schmidt A, Barberis C. In: Progress in Endocrinology. Imura H, Shizume K, editors. Elsevier: Amsterdam; 1988. pp. 1183–1189.
-
- Birnbaumer M, Seibold A, Gilbert S, Ishido M, Barberis C, Antaramian A, Brabet P, Rosenthal W. Nature. 1992;357:333. - PubMed
-
- Kimura T, Tanizawa O, Mori K, Brownstein MJ, Okayama H. Nature. 1992;356:526. - PubMed
-
- Morel A, O'Carroll AM, Brownstein MJ, Lolait SJ. Nature. 1992;356:523. - PubMed
-
- Sugimoto T, Saito M, Mochizuki S, Watanabe Y, Hashimoto S, Kawashima H. J Biol Chem. 1994;269:27088. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases

