Deficiency in Na,K-ATPase alpha isoform genes alters spatial learning, motor activity, and anxiety in mice
- PMID: 17234593
- PMCID: PMC6672804
- DOI: 10.1523/JNEUROSCI.4464-06.2007
Deficiency in Na,K-ATPase alpha isoform genes alters spatial learning, motor activity, and anxiety in mice
Abstract
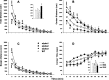
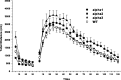
Several disorders have been associated with mutations in Na,K-ATPase alpha isoforms (rapid-onset dystonia parkinsonism, familial hemiplegic migraine type-2), as well as reduction in Na,K-ATPase content (depression and Alzheimer's disease), thereby raising the issue of whether haploinsufficiency or altered enzymatic function contribute to disease etiology. Three isoforms are expressed in the brain: the alpha1 isoform is found in many cell types, the alpha2 isoform is predominantly expressed in astrocytes, and the alpha3 isoform is exclusively expressed in neurons. Here we show that mice heterozygous for the alpha2 isoform display increased anxiety-related behavior, reduced locomotor activity, and impaired spatial learning in the Morris water maze. Mice heterozygous for the alpha3 isoform displayed spatial learning and memory deficits unrelated to differences in cued learning in the Morris maze, increased locomotor activity, an increased locomotor response to methamphetamine, and a 40% reduction in hippocampal NMDA receptor expression. In contrast, heterozygous alpha1 isoform mice showed increased locomotor response to methamphetamine and increased basal and stimulated corticosterone in plasma. The learning and memory deficits observed in the alpha2 and alpha3 heterozygous mice reveal the Na,K-ATPase to be an important factor in the functioning of pathways associated with spatial learning. The neurobehavioral changes seen in heterozygous mice suggest that these mouse models may be useful in future investigations of the associated human CNS disorders.
Figures







References
-
- Anagnostopoulos AV, Mobraaten LE, Sharp JJ, Davisson MT. Transgenic and knockout databases: behavioral profiles of mouse mutants. Physiol Behav. 2001;73:675–689. - PubMed
-
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus: memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. - PubMed
-
- Blasi JM, Cena V, Gonzalez-Garcia C, Marsal J, Solsona C. Ouabain induces acetylcholine release from pure cholinergic synaptosomes independently of extracellular calcium concentration. Neurochem Res. 1988;13:1035–1041. - PubMed
-
- Bolivar V, Cook M, Flaherty L. List of transgenic and knockout mice: behavioral profiles. Mamm Genome. 2000;11:260–274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
