Roles of subthreshold calcium current and sodium current in spontaneous firing of mouse midbrain dopamine neurons
- PMID: 17234596
- PMCID: PMC6672803
- DOI: 10.1523/JNEUROSCI.4341-06.2007
Roles of subthreshold calcium current and sodium current in spontaneous firing of mouse midbrain dopamine neurons
Abstract
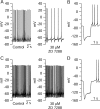
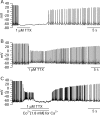
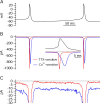
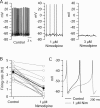
We used a preparation of acutely dissociated neurons to quantify the ionic currents driving the spontaneous firing of substantia nigra pars compacta neurons, isolated from transgenic mice in which the tyrosine hydroxylase promoter drives expression of human placental alkaline phosphatase (PLAP) on the outer surface of the cell membrane. Dissociated neurons identified by fluorescent antibodies to PLAP showed firing properties similar to those of dopaminergic neurons in brain slice, including rhythmic spontaneous firing of broad action potentials and, in some cells, rhythmic oscillatory activity in the presence of tetrodotoxin (TTX). Spontaneous activity in TTX had broader, smaller spikes than normal pacemaking and was stopped by removal of external calcium. Normal pacemaking was also consistently silenced by replacement of external calcium by cobalt and was slowed by more specific calcium channel blockers. Nimodipine produced a slowing of pacemaking frequency. Pacemaking was also slowed by the P/Q-channel blocker omega-Aga-IVA, but the N-type channel blocker omega-conotoxin GVIA had no effect. In voltage-clamp experiments, using records of pacemaking as command voltage, cobalt-sensitive current and TTX-sensitive current were both sizeable at subthreshold voltages between spikes. Cobalt-sensitive current was consistently larger than TTX-sensitive current at interspike voltages from -70 to -50 mV, with TTX-sensitive current larger at voltages positive to -45 mV. These results support previous evidence for a major role of voltage-dependent calcium channels in driving pacemaking of midbrain dopamine neurons and suggest that multiple calcium channel types contribute to this function. The results also show a significant contribution of subthreshold TTX-sensitive sodium current.
Figures





References
-
- Beurrier C, Bioulac B, Hammond C. Slowly inactivating sodium current (I(NaP)) underlies single-spike activity in rat subthalamic neurons. J Neurophysiol. 2000;83:1951–1957. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
