Reliable long-lasting depression interacts with variable short-term facilitation to determine corticostriatal paired-pulse plasticity in young rats
- PMID: 17234703
- PMCID: PMC2075419
- DOI: 10.1113/jphysiol.2006.115790
Reliable long-lasting depression interacts with variable short-term facilitation to determine corticostriatal paired-pulse plasticity in young rats
Abstract
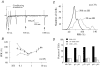
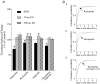
Synaptic plasticity at corticostraital synapses is proposed to fine tune movment and improve motor skills. We found paired-pulse plasticity at corticostriatal synapses reflected variably expressed short-term facilitation blended with a consistent background of longer-lasting depression. Presynaptic modulation via neuotransmitter receptor activation was ruled out as a mechanism for long-lasting paired-pulse depression by examining the effect of selective receptor antagonists. EPSC amplitude and paired-pulse plasticity, however, was influenced by block of D2 dopamine receptors. Block of glutamate transport with l-transdicarboxylic acid (PDC) reduced EPSCs, possibly through a mechanism of AMPA receptor desensitization. Removal of AMPA receptor desensitization with cyclothiazide reduced the paired-pulse depression at long-duration interstimulus intervals (ISIs), indicating that AMPA receptor desensitization participates in corticostriatal paired-pulse plasticity. The low-affinity glutamate receptor antagonist cis-2,3-piperidine dicarboxylic acid (PDA) increased paired-pulse depression, suggesting that a presynaptic component also exists for long-lasting paired-pulse depression. Low Ca(2+)-high Mg(2+) or BAPTA-AM dramatically reduced the amplitude of corticostriatal EPSCs and both manipulations increased the expression of facilitation and, to a lesser extent, they reduced long-lasting paired-pulse depression. EGTA-AM produced a smaller reduction in EPSC amplitude and it did not alter paired-pulse facilitation, but in contrast to low Ca(2+) and BAPTA-AM, EGTA-AM increased long-lasting paired-pulse depression. These experiments suggest that facilitation and depression are sensitive to vesicle depletion, which is dependent upon changes in peak Ca(2+) (i.e. low Ca(2+)-high Mg(2+) or BAPTA-AM). In addition, the action of EGTA-AM suggests that basal Ca(2+) regulates the recovery from long-lasting paired-pulse depression, possibly thourgh a Ca(2+)-sensitive process of vesicle delivery.
Figures
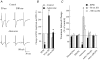
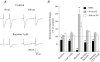


References
-
- Aghajanian GK, Rasmussen K. Intracellular studies in the fa facial nucleus illustratiny a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3:331–338. - PubMed
-
- Akopian G, Musleh W, Smith R, Walsh JP. Functional state of presynaptic terminals influences the expression of short- and long-term plasticity at corticostriatal synapses. Synapse. 2000;38:271–280. - PubMed
-
- Akopian G, Walsh JP. Corticostriatal paired-pulse potentiation produced by voltage-dependent increases in NMDA receptor and L-type Ca2+ channel activation. J Neurophysiol. 2002;87:157–165. - PubMed
-
- Akopian G, Walsh JP. Reduced expression of short- and long-term facilitation at aged corticostriatal synapses. Synapse. 2006;60:223–238. - PubMed
-
- Ali AB, Nelson C. Distinct Ca2+ channels mediate transmitter release at excitatory synapses displaying different dynamic properties in rat neocortex. Cerebral Cortex. 2006;16:386–393. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

