A functional study of plasma-membrane calcium-pump isoform 2 mutants causing digenic deafness
- PMID: 17234811
- PMCID: PMC1785272
- DOI: 10.1073/pnas.0609775104
A functional study of plasma-membrane calcium-pump isoform 2 mutants causing digenic deafness
Abstract
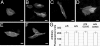
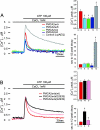
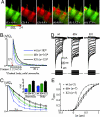
Ca2+ enters the stereocilia of hair cells through mechanoelectrical transduction channels opened by the deflection of the hair bundle and is exported back to endolymph by an unusual splicing isoform (w/a) of plasma-membrane calcium-pump isoform 2 (PMCA2). Ablation or missense mutations of the pump cause deafness, as described for the G283S mutation in the deafwaddler (dfw) mouse. A deafness-inducing missense mutation of PMCA2 (G293S) has been identified in a human family. The family also was screened for mutations in cadherin 23, which accentuated hearing loss in a previously described human family with a PMCA2 mutation. A T1999S substitution was detected in the cadherin 23 gene of the healthy father and affected son but not in that of the unaffected mother, who presented instead the PMCA2 mutation. The w/a isoform was overexpressed in CHO cells. At variance with the other PMCA2 isoforms, it became activated only marginally when exposed to a Ca2+ pulse. The G293S and G283S mutations delayed the dissipation of Ca2+ transients induced in CHO cells by InsP3. In organotypic cultures, Ca2+ imaging of vestibular hair cells showed that the dissipation of stereociliary Ca2+ transients induced by Ca2+ uncaging was compromised in the dfw and PMCA2 knockout mice, as was the sensitivity of the mechanoelectrical transduction channels to hair bundle displacement in cochlear hair cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

