Cyclic AMP-dependent protein kinase and Epac mediate cyclic AMP responses in pancreatic acini
- PMID: 17234888
- PMCID: PMC2975017
- DOI: 10.1152/ajpgi.00478.2005
Cyclic AMP-dependent protein kinase and Epac mediate cyclic AMP responses in pancreatic acini
Abstract
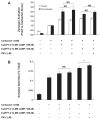
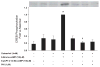
The pancreatic acinar cell has several phenotypic responses to cAMP agonists. At physiological concentrations of the muscarinic agonist carbachol (1 microM) or the CCK analog caerulein (100 pM), ligands that increase cytosolic Ca(2+), cAMP acts synergistically to enhance secretion. Supraphysiological concentrations of carbachol (1 mM) or caerulein (100 nM) suppress secretion and cause intracellular zymogen activation; cAMP enhances both zymogen activation and reverses the suppression of secretion. In addition to stimulating cAMP-dependent protein kinase (PKA), recent studies using cAMP analogs that lack a PKA response have shown that cAMP can also act through the cAMP-binding protein, Epac (exchange protein directly activated by cyclic AMP). The roles of PKA and Epac in cAMP responses were examined in isolated pancreatic acini. The activation of both cAMP-dependent pathways or the selective activation of Epac was found to enhance amylase secretion induced by physiological and supraphysiological concentrations of the muscarinic agonist carbachol. Similarly, activation of both PKA or the specific activation of Epac enhanced carbachol-induced activation of trypsinogen and chymotrypsinogen. Disorganization of the apical actin cytoskeleton has been linked to the decreased secretion observed with supraphysiological concentrations of carbachol and caerulein. Although stimulation of PKA and Epac or Epac alone could largely overcome the decreased secretion observed with either supraphysiological carbachol or caerulein, stimulation of cAMP pathways did not reduce the disorganization of the apical cytoskeleton. These studies demonstrate that PKA and Epac pathways are coupled to both secretion and zymogen activation in the pancreatic acinar cell.
Figures




Similar articles
-
Effects of increased intracellular cAMP on carbachol-stimulated zymogen activation, secretion, and injury in the pancreatic acinar cell.Am J Physiol Gastrointest Liver Physiol. 2005 Feb;288(2):G235-43. doi: 10.1152/ajpgi.00334.2004. Epub 2004 Sep 30. Am J Physiol Gastrointest Liver Physiol. 2005. PMID: 15458924 Free PMC article.
-
Cyclic AMP-dependent protein kinase A and EPAC mediate VIP and secretin stimulation of PAK4 and activation of Na+,K+-ATPase in pancreatic acinar cells.Am J Physiol Gastrointest Liver Physiol. 2019 Feb 1;316(2):G263-G277. doi: 10.1152/ajpgi.00275.2018. Epub 2018 Dec 6. Am J Physiol Gastrointest Liver Physiol. 2019. PMID: 30520694 Free PMC article.
-
Rap1 activation plays a regulatory role in pancreatic amylase secretion.J Biol Chem. 2008 Aug 29;283(35):23884-94. doi: 10.1074/jbc.M800754200. Epub 2008 Jun 24. J Biol Chem. 2008. PMID: 18577515 Free PMC article.
-
Epac-selective cAMP analogs: new tools with which to evaluate the signal transduction properties of cAMP-regulated guanine nucleotide exchange factors.Cell Signal. 2008 Jan;20(1):10-20. doi: 10.1016/j.cellsig.2007.07.009. Epub 2007 Jul 25. Cell Signal. 2008. PMID: 17716863 Free PMC article. Review.
-
Epac: defining a new mechanism for cAMP action.Annu Rev Pharmacol Toxicol. 2010;50:355-75. doi: 10.1146/annurev.pharmtox.010909.105714. Annu Rev Pharmacol Toxicol. 2010. PMID: 20055708 Review.
Cited by
-
Secretin is not necessary for exocrine pancreatic development and growth in mice.Am J Physiol Gastrointest Liver Physiol. 2011 Nov;301(5):G791-8. doi: 10.1152/ajpgi.00245.2011. Epub 2011 Aug 18. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21852360 Free PMC article.
-
Intracellular cAMP Sensor EPAC: Physiology, Pathophysiology, and Therapeutics Development.Physiol Rev. 2018 Apr 1;98(2):919-1053. doi: 10.1152/physrev.00025.2017. Physiol Rev. 2018. PMID: 29537337 Free PMC article. Review.
-
Molecular basis for pancreatitis.Curr Opin Gastroenterol. 2008 Sep;24(5):580-5. doi: 10.1097/MOG.0b013e32830b10e6. Curr Opin Gastroenterol. 2008. PMID: 19122498 Free PMC article. Review.
-
VIP and muscarinic synergistic mucin secretion by salivary mucous cells is mediated by enhanced PKC activity via VIP-induced release of an intracellular Ca2+ pool.Pflugers Arch. 2020 Mar;472(3):385-403. doi: 10.1007/s00424-020-02348-7. Epub 2020 Jan 13. Pflugers Arch. 2020. PMID: 31932898 Free PMC article.
-
Ca²⁺-regulated secretory granule exocytosis in pancreatic and parotid acinar cells.Cell Calcium. 2014 Jun;55(6):369-75. doi: 10.1016/j.ceca.2014.03.003. Epub 2014 Mar 28. Cell Calcium. 2014. PMID: 24742357 Free PMC article. Review.
References
-
- Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol. 2003;4:733–738. - PubMed
-
- Bos JL. Linking Rap to cell adhesion. Curr Opin Cell Biol. 2005;17:123–128. - PubMed
-
- Burnham DB, Williams JA. Effects of high concentrations of secretagogues on the morphology and secretory activity of the pancreas: a role for microfilaments. Cell Tissue Res. 1982;222:201–212. - PubMed
-
- De Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

