Selection of embryonic stem cell-derived enhanced green fluorescent protein-positive dopamine neurons using the tyrosine hydroxylase promoter is confounded by reporter gene expression in immature cell populations
- PMID: 17234989
- PMCID: PMC2614084
- DOI: 10.1634/stemcells.2006-0540
Selection of embryonic stem cell-derived enhanced green fluorescent protein-positive dopamine neurons using the tyrosine hydroxylase promoter is confounded by reporter gene expression in immature cell populations
Abstract
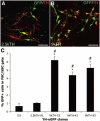
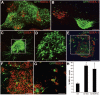
Transplantation of mouse embryonic stem (mES) cells can restore function in Parkinson disease models, but can generate teratomas. Purification of dopamine neurons derived from embryonic stem cells by fluorescence-activated cell sorting (FACS) could provide a functional cell population for transplantation while eliminating the risk of teratoma formation. Here we used the tyrosine hydroxylase (TH) promoter to drive enhanced green fluorescent protein (eGFP) expression in mES cells. First, we evaluated 2.5-kilobase (kb) and 9-kb TH promoter fragments and showed that clones generated using the 9-kb fragment produced significantly more eGFP+/TH+ neurons. We selected the 9-kb TH clone with the highest eGFP/TH overlap for further differentiation, FACS, and transplantation experiments. Grafts contained large numbers of eGFP+ dopamine neurons of an appropriate phenotype. However, there were also numerous eGFP+ cells that did not express TH and did not have a neuronal morphology. In addition, we found cells in the grafts representing all three germ layers. Based on these findings, we examined the expression of stem cell markers in our eGFP+ population. We found that a majority of eGFP+ cells were stage-specific embryonic antigen-positive (SSEA-1+) and that the genetically engineered clones contained more SSEA-1+ cells after differentiation than the original D3 mES cells. By negative selection of SSEA-1, we could isolate a neuronal eGFP+ population of high purity. These results illustrate the complexity of using genetic selection to purify mES cell-derived dopamine neurons and provide a comprehensive analysis of cell selection strategies based on tyrosine hydroxylase expression. Disclosure of potential conflicts of interest is found at the end of this article.
Figures





References
-
- Kim JH, Auerbach JM, Rodriguez-Gomez JA, et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418:50–56. - PubMed
-
- Barberi T, Klivenyi P, Calingasan NY, et al. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat Biotechnol. 2003;21:1200–1207. - PubMed
-
- Ben-Hur T, Idelson M, Khaner H, et al. Transplantation of human embryonic stem cell-derived neural progenitors improves behavioral deficit in Parkinsonian rats. STEM CELLS. 2004;22:1246–1255. - PubMed
-
- Arnhold S, Klein H, Semkova I, et al. Neurally selected embryonic stem cells induce tumor formation after long-term survival following engraftment into the subretinal space. Invest Ophthalmol Vis Sci. 2004;45:4251–4255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

