PATJ regulates directional migration of mammalian epithelial cells
- PMID: 17235357
- PMCID: PMC1796763
- DOI: 10.1038/sj.embor.7400890
PATJ regulates directional migration of mammalian epithelial cells
Abstract
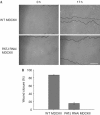
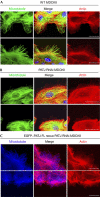
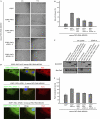
Directional migration is important in wound healing by epithelial cells. Recent studies have shown that polarity proteins such as mammalian Partitioning-defective 6 (Par6), atypical protein kinase C (aPKC) and mammalian Discs large 1 (Dlg1) are crucial not only for epithelial apico-basal polarity, but also for directional movement. Here, we show that the protein associated with Lin seven 1 (PALS1)-associated tight junction protein (PATJ), another evolutionarily conserved polarity protein, is also required for directional migration by using a wound-induced migration assay. In addition, we found that aPKC and Par3 localize to the leading edge during migration of epithelia and that PATJ regulates their localization. Furthermore, our results show that microtubule-organizing centre orientation is disrupted in PATJ RNA interference (RNAi) MDCKII (Madin-Darby canine kidney II) cells during migration. Together, our data indicate that PATJ controls directional migration by regulating the localization of aPKC and Par3 to the leading edge. The migration defect in PATJ RNAi cells seems to be due to the disorganization of the microtubule network induced by mislocalization of polarity proteins.
Figures

References
-
- Bazzoni G, Martinez-Estrada OM, Orsenigo F, Cordenonsi M, Citi S, Dejana E (2000) Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J Biol Chem 275: 20520–20526 - PubMed
-
- Berrueta L, Tirnauer JS, Schuyler SC, Pellman D, Bierer BE (1999) The APC-associated protein EB1 associates with components of the dynactin complex and cytoplasmic dynein intermediate chain. Curr Biol 9: 425–428 - PubMed
-
- Chen X, Macara IG (2005) Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol 7: 262–269 - PubMed
-
- Craig AM, Banker G (1994) Neuronal polarity. Annu Rev Neurosci 17: 267–310 - PubMed
-
- Ebnet K, Schulz CU, Meyer Zu Brickwedde MK, Pendl GG, Vestweber D (2000) Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J Biol Chem 275: 27979–27988 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

