MEL-28/ELYS is required for the recruitment of nucleoporins to chromatin and postmitotic nuclear pore complex assembly
- PMID: 17235358
- PMCID: PMC1796766
- DOI: 10.1038/sj.embor.7400889
MEL-28/ELYS is required for the recruitment of nucleoporins to chromatin and postmitotic nuclear pore complex assembly
Abstract
The metazoan nuclear envelope (NE) breaks down and re-forms during each cell cycle. Nuclear pore complexes (NPCs), which allow nucleocytoplasmic transport during interphase, assemble into the re-forming NE at the end of mitosis. Using in vitro NE assembly, we show that the vertebrate homologue of MEL-28 (maternal effect lethal), a recently discovered NE component in Caenorhabditis elegans, functions in postmitotic NPC assembly. MEL-28 interacts with the Nup107-160 complex (Nup for nucleoporin), an important building block of the NPC, and is essential for the recruitment of the Nup107-160 complex to chromatin. We suggest that MEL-28 acts as a seeding point for NPC assembly.
Figures
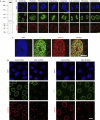
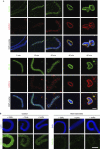
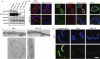
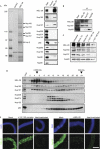
References
-
- Antonin W, Franz C, Haselmann U, Antony C, Mattaj IW (2005) The integral membrane nucleoporin pom121 functionally links nuclear pore complex assembly and nuclear envelope formation. Mol Cell 17: 83–92 - PubMed
-
- Brachner A, Reipert S, Foisner R, Gotzmann J (2005) LEM2 is a novel MAN1-related inner nuclear membrane protein associated with A-type lamins. J Cell Sci 118: 5797–5810 - PubMed
-
- Fernandez AG, Piano F (2006) Network analysis of C. elegans early embryogenesis identifies MEL-28 as a central coordinator of chromatin maintenance and nuclear envelope function. Curr Biol 16: 1757–1763 - PubMed
-
- Galy V, Askjaer P, Franz C, López-Iglesias C, Mattaj IW (2006) MEL-28, a novel nuclear envelope and kinetochore protein essential for zygotic nuclear envelope assembly in C. elegans. Curr Biol 16: 1748–1756 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

