Vascular endothelial growth factor and dexamethasone release from nonfouling sensor coatings affect the foreign body response
- PMID: 17236219
- PMCID: PMC4070388
- DOI: 10.1002/jbm.a.31088
Vascular endothelial growth factor and dexamethasone release from nonfouling sensor coatings affect the foreign body response
Abstract
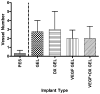
Vascular endothelial growth factor (VEGF) and dexamethasone (DX) release from hydrogel coatings were examined as a means to modify tissue inflammation and induce angiogenesis. Antibiofouling hydrogels for implantable glucose sensor coatings were prepared from 2-hydroxyethyl methacrylate, N-vinyl pyrrolidinone, and polyethylene glycol. Microdialysis sampling was used to test the effect of the hydrogel coating on glucose recovery. VEGF-releasing hydrogel-coated fibers increased vascularity and inflammation in the surrounding tissue after 2 weeks of implantation compared to hydrogel-coated fibers. DX-releasing hydrogel-coated fibers reduced inflammation compared to hydrogel-coated fibers and had reduced capsule vascularity compared to VEGF-releasing hydrogel-coated fibers. Hydrogels that released both VEGF and DX simultaneously also showed reduced inflammation at 2 weeks implantation; however, no enhanced vessel formation was observed indicating that the DX diminished the VEGF effect. At 6 weeks, there were no detectable differences between drug-releasing hydrogel-coated fibers and control fibers. From this study, hydrogel drug release affected initial events of the foreign body response with DX inhibiting VEGF, but once the drug depot was exhausted these effects disappeared.
(c) 2007 Wiley Periodicals, Inc.
Figures






Similar articles
-
In vitro characterization of vascular endothelial growth factor and dexamethasone releasing hydrogels for implantable probe coatings.Biomaterials. 2005 Jun;26(16):3285-97. doi: 10.1016/j.biomaterials.2004.07.069. Biomaterials. 2005. PMID: 15603824
-
Sequential delivery of dexamethasone and VEGF to control local tissue response for carbon nanotube fluorescence based micro-capillary implantable sensors.Biomaterials. 2009 Feb;30(4):622-31. doi: 10.1016/j.biomaterials.2008.09.052. Epub 2008 Nov 8. Biomaterials. 2009. PMID: 18996588
-
Multiple tissue response modifiers to promote angiogenesis and prevent the foreign body reaction around subcutaneous implants.J Control Release. 2015 Sep 28;214:103-11. doi: 10.1016/j.jconrel.2015.07.021. Epub 2015 Jul 26. J Control Release. 2015. PMID: 26216396
-
Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis.J Control Release. 2007 Jan 22;117(1):68-79. doi: 10.1016/j.jconrel.2006.10.013. Epub 2006 Oct 19. J Control Release. 2007. PMID: 17169457
-
Biomaterials/tissue interactions: possible solutions to overcome foreign body response.AAPS J. 2010 Jun;12(2):188-96. doi: 10.1208/s12248-010-9175-3. Epub 2010 Feb 9. AAPS J. 2010. PMID: 20143194 Free PMC article. Review.
Cited by
-
Biomechanics of the sensor-tissue interface-effects of motion, pressure, and design on sensor performance and the foreign body response-part I: theoretical framework.J Diabetes Sci Technol. 2011 May 1;5(3):632-46. doi: 10.1177/193229681100500317. J Diabetes Sci Technol. 2011. PMID: 21722578 Free PMC article. Review.
-
A remotely operated drug delivery system with an electrolytic pump and a thermo-responsive valve.Biomicrofluidics. 2015 Jul 22;9(5):052608. doi: 10.1063/1.4927436. eCollection 2015 Sep. Biomicrofluidics. 2015. PMID: 26339328 Free PMC article.
-
Anti-inflammatory polymeric coatings for implantable biomaterials and devices.J Diabetes Sci Technol. 2008 Nov;2(6):984-94. doi: 10.1177/193229680800200628. J Diabetes Sci Technol. 2008. PMID: 19885288 Free PMC article.
-
Crosslinked basement membrane-based coatings enhance glucose sensor function and continuous glucose monitoring in vivo.J Biomed Mater Res A. 2018 Jan;106(1):7-16. doi: 10.1002/jbm.a.36206. Epub 2017 Sep 19. J Biomed Mater Res A. 2018. PMID: 28875571 Free PMC article.
-
Chronic inflammatory responses to microgel-based implant coatings.J Biomed Mater Res A. 2010 Jul;94(1):252-8. doi: 10.1002/jbm.a.32669. J Biomed Mater Res A. 2010. PMID: 20166218 Free PMC article.
References
-
- Moussy F, Reichert WM. Biomaterials community examines biosensor biocompatibility. Diabetes Technol Ther. 2000;2:473–477. - PubMed
-
- Wilson GS, Gifford R. Biosensors for real-time in vivo measurements. Biosens Bioelectron. 2005;20:2388–2403. - PubMed
-
- Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, Johnson RC. Neovascularization of synthetic membranes directed by membrane microarchitecture. J Biomed Mater Res. 1995;29:1517–1524. - PubMed
-
- Ratner BD, Bryant SJ. Biomaterials: Where we have been and where we are going. Annu Rev Biomed Eng. 2004;6:41–75. - PubMed
-
- Wisniewski N, Moussy F, Reichert WM. Characterization of implantable biosensor membrane biofouling. Fresenius J Anal Chem. 2000;366:611–621. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

