The sodium-coupled neutral amino acid transporter SNAT2 mediates an anion leak conductance that is differentially inhibited by transported substrates
- PMID: 17237199
- PMCID: PMC1864845
- DOI: 10.1529/biophysj.106.100776
The sodium-coupled neutral amino acid transporter SNAT2 mediates an anion leak conductance that is differentially inhibited by transported substrates
Abstract
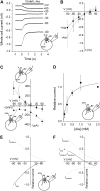
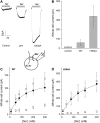
The sodium-coupled neutral amino acid transporter SNAT2 mediates cellular uptake of glutamine and other small, neutral amino acids. Here, we report the existence of a leak anion pathway associated with SNAT2. The leak anion conductance was increased by, but did not require the presence of, extracellular sodium. The transported substrates L-alanine, L-glutamine, and alpha-(methylamino)isobutyrate inhibited the anion leak conductance, each with different potency. A transporter with the mutation H-304A did not catalyze alanine transport but still catalyzed anion leak current, demonstrating that substrate transport is not required for anion current inhibition. Both the substrate and Na+ were able to bind to the SNAT2H-304A transporter normally. The selectivity sequence of the SNAT2H-304A anion conductance was SCN->>NO3->I->Br->Cl->Mes-. Anion flux mediated by the more hydrophobic anion SCN- was not saturable, whereas nitrate flux demonstrated saturation kinetics with an apparent Km of 29 mM. SNAT2, which belongs to the SLC38 family of transporters, has to be added to the growing number of secondary, Na+-coupled transporters catalyzing substrate-gated or leak anion conductances. Therefore, we can speculate that such anion-conducting pathways are general features of Na+-transporting systems.
Figures


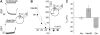
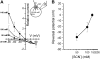
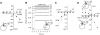

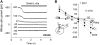

References
-
- Johnson, L. W., and C. H. Smith. 1988. Neutral amino acid transport systems of microvillous membrane of human placenta. Am. J. Physiol. 254:C773–C780. - PubMed
-
- Sugawara, M., T. Nakanishi, Y. J. Fei, W. Huang, M. E. Ganapathy, F. H. Leibach, and V. Ganapathy. 2000. Cloning of an amino acid transporter with functional characteristics and tissue expression pattern identical to that of system A. J. Biol. Chem. 275:16473–16477. - PubMed
-
- Bode, B. P. 2001. Recent molecular advances in mammalian glutamine transport. J. Nutr. 131:2475S–2485S. - PubMed
-
- Yao, D., B. Mackenzie, H. Ming, H. Varoqui, H. Zhu, M. A. Hediger, and J. D. Erickson. 2000. A novel system A isoform mediating Na+/neutral amino acid cotransport. J. Biol. Chem. 275:22790–22797. - PubMed
-
- Shotwell, M. A., D. W. Jayme, M. S. Kilberg, and D. L. Oxender. 1981. Neutral amino acid transport systems in Chinese hamster ovary cells. J. Biol. Chem. 256:5422–5427. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

