Thermodynamic analysis of the lipopolysaccharide-dependent resistance of gram-negative bacteria against polymyxin B
- PMID: 17237210
- PMCID: PMC1831710
- DOI: 10.1529/biophysj.106.095711
Thermodynamic analysis of the lipopolysaccharide-dependent resistance of gram-negative bacteria against polymyxin B
Abstract
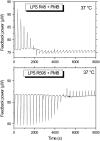
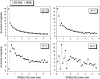
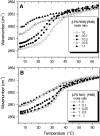
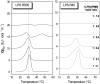
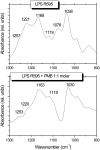
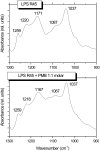
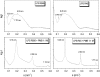
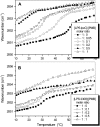
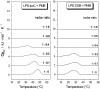
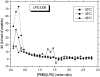
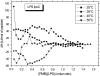
Cationic antimicrobial cationic peptides (CAMP) have been found in recent years to play a decisive role in hosts' defense against microbial infection. They have also been investigated as a new therapeutic tool, necessary in particular due to the increasing resistance of microbiological populations to antibiotics. The structural basis of the activity of CAMPs has only partly been elucidated and may comprise quite different mechanism at the site of the bacterial cell membranes or in their cytoplasm. Polymyxin B (PMB) is a CAMP which is effective in particular against Gram-negative bacteria and has been well studied with the aim to understand its interaction with the outer membrane or isolated membrane components such as lipopolysaccharide (LPS) and to define the mechanism by which the peptides kill bacteria or neutralize LPS. Since PMB resistance of bacteria is a long-known phenomenon and is attributed to structural changes in the LPS moiety of the respective bacteria, we have performed a thermodynamic and biophysical analysis to get insights into the mechanisms of various LPS/PMB interactions in comparison to LPS from sensitive strains. In isothermal titration calorimetric (ITC) experiments considerable differences of PMB binding to sensitive and resistant LPS were found. For sensitive LPS the endothermic enthalpy change in the gel phase of the hydrocarbon chains converts into an exothermic reaction in the liquid crystalline phase. In contrast, for resistant LPS the binding enthalpy change remains endothermic in both phases. As infrared data show, these differences can be explained by steric changes in the headgroup region of the respective LPS.
Figures
Similar articles
-
Liquid crystalline bacterial outer membranes are critical for antibiotic susceptibility.Proc Natl Acad Sci U S A. 2018 Aug 7;115(32):E7587-E7594. doi: 10.1073/pnas.1803975115. Epub 2018 Jul 23. Proc Natl Acad Sci U S A. 2018. PMID: 30037998 Free PMC article.
-
Titration calorimetric studies to elucidate the specificity of the interactions of polymyxin B with lipopolysaccharides and lipid A.Biochem J. 1996 Apr 15;315 ( Pt 2)(Pt 2):679-86. doi: 10.1042/bj3150679. Biochem J. 1996. PMID: 8615847 Free PMC article.
-
Probing the properties of lipopolysaccharide monolayers and their interaction with the antimicrobial peptide polymyxin B by atomic force microscopy.Langmuir. 2005 Jul 19;21(15):6970-8. doi: 10.1021/la048218c. Langmuir. 2005. PMID: 16008411
-
NMR Structures and Interactions of Antimicrobial Peptides with Lipopolysaccharide: Connecting Structures to Functions.Curr Top Med Chem. 2016;16(1):4-15. doi: 10.2174/1568026615666150703121943. Curr Top Med Chem. 2016. PMID: 26139110 Review.
-
Polymyxin B: an ode to an old antidote for endotoxic shock.Mol Biosyst. 2005 Sep;1(3):213-22. doi: 10.1039/b500756a. Epub 2005 Jul 29. Mol Biosyst. 2005. PMID: 16880985 Review.
Cited by
-
Biophysical mechanisms of the neutralization of endotoxins by lipopolyamines.Open Biochem J. 2013 Sep 30;7:82-93. doi: 10.2174/1874091X01307010082. eCollection 2013. Open Biochem J. 2013. PMID: 24133550 Free PMC article.
-
NMR structure of pardaxin, a pore-forming antimicrobial peptide, in lipopolysaccharide micelles: mechanism of outer membrane permeabilization.J Biol Chem. 2010 Feb 5;285(6):3883-3895. doi: 10.1074/jbc.M109.065672. Epub 2009 Dec 3. J Biol Chem. 2010. PMID: 19959835 Free PMC article.
-
Lipopolysaccharide neutralization by antimicrobial peptides: a gambit in the innate host defense strategy.J Innate Immun. 2012;4(4):327-36. doi: 10.1159/000336713. Epub 2012 Mar 21. J Innate Immun. 2012. PMID: 22441679 Free PMC article. Review.
-
Polymyxin B induces lysis of marine pseudoalteromonads.Antimicrob Agents Chemother. 2007 Nov;51(11):3908-14. doi: 10.1128/AAC.00449-07. Epub 2007 Aug 20. Antimicrob Agents Chemother. 2007. PMID: 17709471 Free PMC article.
-
Design, synthesis, and evaluation of a new fluorescent probe for measuring polymyxin-lipopolysaccharide binding interactions.Anal Biochem. 2011 Feb 15;409(2):273-83. doi: 10.1016/j.ab.2010.10.033. Epub 2010 Nov 2. Anal Biochem. 2011. PMID: 21050838 Free PMC article.
References
-
- Velasco, J., J. A. Bengoechea, K. Brandenburg, B. Lindner, U. Seydel, D. González, U. Zähringer, E. Moreno, and I. Moriyón. 2000. Brucella abortus and its closest phylogenetic relative, Ochrobactrum spp., differ in outer membrane permeability and cationic peptide resistance. Infect. Immun. 68:3210–3218. - PMC - PubMed
-
- Wiese, A., M. Münstermann, T. Gutsmann, B. Lindner, K. Kawahara, U. Zähringer, and U. Seydel. 1998. Molecular mechanisms of polymyxin B-membrane interactions: direct correlation between surface charge density and self-promoted uptake. J. Membr. Biol. 162:127–138. - PubMed
-
- Schromm, A. B., K. Brandenburg, H. Loppnow, A. P. Moran, M. H. J. Koch, E. Th. Rietschel, and U. Seydel. 2000. Biological activities of lipopolysaccharides are determined by the shape of their lipid A portion. Eur. J. Biochem. 267:2008–2013. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

