Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted
- PMID: 17237343
- PMCID: PMC2656382
- DOI: 10.1152/physrev.00022.2006
Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted
Abstract
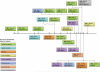
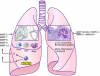
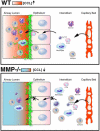
The matrix metalloproteinases (MMPs), a family of 25 secreted and cell surface-bound neutral proteinases, process a large array of extracellular and cell surface proteins under normal and pathological conditions. MMPs play critical roles in lung organogenesis, but their expression, for the most part, is downregulated after generation of the alveoli. Our knowledge about the resurgence of the MMPs that occurs in most inflammatory diseases of the lung is rapidly expanding. Although not all members of the MMP family are found within the lung tissue, many are upregulated during the acute and chronic phases of these diseases. Furthermore, potential MMP targets in the lung include all structural proteins in the extracellular matrix (ECM), cell adhesion molecules, growth factors, cytokines, and chemokines. However, what is less known is the role of MMP proteolysis in modulating the function of these substrates in vivo. Because of their multiplicity and substantial substrate overlap, MMPs are thought to have redundant functions. However, as we explore in this review, such redundancy most likely evolved as a necessary compensatory mechanism given the critical regulatory importance of MMPs. While inhibition of MMPs has been proposed as a therapeutic option in a variety of inflammatory lung conditions, a complete understanding of the biology of these complex enzymes is needed before we can reasonably consider them as therapeutic targets.
Figures



References
-
- Mechanisms and limits of induced postnatal lung growth. Am J Respir Crit Care Med. 2004;170:319–343. - PubMed
-
- Adamson IYR, Vincent R, Bakowska J. Differential production of metalloproteinases after instilling various urban air particle samples to rat lung. Exp Lung Res. 2003;29:375–388. - PubMed
-
- Ahokas K, Lohi J, Illman SA, Llano E, Elomaa O, Impola U, Karjalainen-Lindsberg ML, Saarialho-Kere U. Matrix metalloproteinase-21 is expressed epithelially during development and in cancer and is up-regulated by transforming growth factor-beta 1 in keratinocytes. Lab Invest. 2003;83:1887–1899. - PubMed
-
- Al-Aqeel AI. Al-Aqeel Sewairi syndrome, a new autosomal recessive disorder with multicentric osteolysis, nodulosis, and arthropathy. The first genetic defect of matrix metalloproteinase 2 gene. Saudi Med J. 2005;26:24–30. - PubMed
-
- Anteby EY, Greenfield C, Natanson-Yaron S, Goldman-Wohl D, Hamani Y, Khudyak V, Ariel I, Yagel S. Vascular endothelial growth factor, epidermal growth factor and fibroblast growth factor-4 and -10 stimulate trophoblast plasminogen activator system and metalloproteinase-9. Mol Hum Reprod. 2004;10:229–235. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

