Flavonoid accumulation in Arabidopsis repressed in lignin synthesis affects auxin transport and plant growth
- PMID: 17237352
- PMCID: PMC1820963
- DOI: 10.1105/tpc.106.044495
Flavonoid accumulation in Arabidopsis repressed in lignin synthesis affects auxin transport and plant growth
Abstract
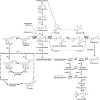
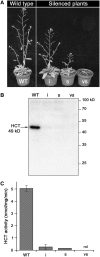
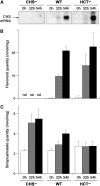
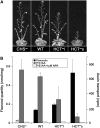
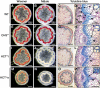
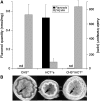
In Arabidopsis thaliana, silencing of hydroxycinnamoyl-CoA shikimate/quinate hydroxycinnamoyl transferase (HCT), a lignin biosynthetic gene, results in a strong reduction of plant growth. We show that, in HCT-silenced plants, lignin synthesis repression leads to the redirection of the metabolic flux into flavonoids through chalcone synthase activity. Several flavonol glycosides and acylated anthocyanin were shown to accumulate in higher amounts in silenced plants. By contrast, sinapoylmalate levels were barely affected, suggesting that the synthesis of that phenylpropanoid compound might be HCT-independent. The growth phenotype of HCT-silenced plants was shown to be controlled by light and to depend on chalcone synthase expression. Histochemical analysis of silenced stem tissues demonstrated altered tracheary elements. The level of plant growth reduction of HCT-deficient plants was correlated with the inhibition of auxin transport. Suppression of flavonoid accumulation by chalcone synthase repression in HCT-deficient plants restored normal auxin transport and wild-type plant growth. By contrast, the lignin structure of the plants simultaneously repressed for HCT and chalcone synthase remained as severely altered as in HCT-silenced plants, with a large predominance of nonmethoxylated H units. These data demonstrate that the reduced size phenotype of HCT-silenced plants is not due to the alteration of lignin synthesis but to flavonoid accumulation.
Figures




References
-
- Atanassova, R., Favet, N., Martz, F., Chabbert, B., Thollier, M.T., Monties, B., Fritig, B., and Legrand, M. (1995). Altered lignin composition in transgenic tobacco expressing O-methyltransferase sequences in sense and antisense orientation. Plant J. 8 465–477.
-
- Benjamins, R., Quint, A., Weijers, D., Hooykaas, P., and Offringa, R. (2001). The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 128 4057–4067. - PubMed
-
- Berleth, T., Mattsson, J., and Hardtke, C.S. (2000). Vascular continuity and auxin signals. Trends Plant Sci. 5 387–393. - PubMed
-
- Blakeslee, J.J., Peer, W.A., and Murphy, A.S. (2005). Auxin transport. Curr. Opin. Plant Biol. 8 494–500. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

