Pyrimethamine as a potential pharmacological chaperone for late-onset forms of GM2 gangliosidosis
- PMID: 17237499
- PMCID: PMC1851921
- DOI: 10.1074/jbc.M609304200
Pyrimethamine as a potential pharmacological chaperone for late-onset forms of GM2 gangliosidosis
Abstract
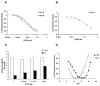
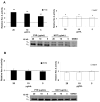
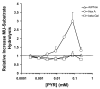
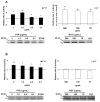
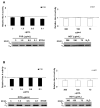
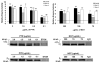
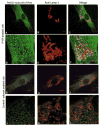
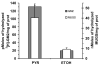
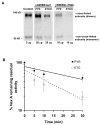
Late-onset GM2 gangliosidosis is composed of two related, autosomal recessive, neurodegenerative diseases, both resulting from deficiency of lysosomal, heterodimeric beta-hexosaminidase A (Hex A, alphabeta). Pharmacological chaperones (PC) are small molecules that can stabilize the conformation of a mutant protein, allowing it to pass the quality control system of the endoplasmic reticulum. To date all successful PCs have also been competitive inhibitors. Screening for Hex A inhibitors in a library of 1040 Food Drug Administration-approved compounds identified pyrimethamine (PYR (2,4-diamino 5-(4-chlorophenyl)-6-ethylpyrimidine)) as the most potent inhibitor. Cell lines from 10 late-onset Tay-Sachs (11 alpha-mutations, 2 novel) and 7 Sandhoff (9 beta-mutations, 4 novel) disease patients, were cultured with PYR at concentrations corresponding to therapeutic doses. Cells carrying the most common late-onset mutation, alphaG269S, showed significant increases in residual Hex A activity, as did all 7 of the beta-mutants tested. Cells responding to PC treatment included those carrying mutants resulting in reduced Hex heat stability and partial splice junction mutations of the inherently less stable alpha-subunit. PYR, which binds to the active site in domain II, was able to function as PC even to domain I beta-mutants. We concluded that PYR functions as a mutation-specific PC, variably enhancing residual lysosomal Hex A levels in late-onset GM2 gangliosidosis patient cells.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

