Control of local actin assembly by membrane fusion-dependent compartment mixing
- PMID: 17237773
- PMCID: PMC4398993
- DOI: 10.1038/ncb1527
Control of local actin assembly by membrane fusion-dependent compartment mixing
Abstract
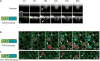
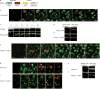
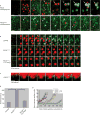
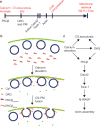
Local actin assembly is associated with sites of exocytosis in processes ranging from phagocytosis to compensatory endocytosis. Here, we examine whether the trigger for actin-coat assembly around exocytosing Xenopus egg cortical granules is 'compartment mixing'--the union of the contents of the plasma membrane with that of the secretory granule membrane. Consistent with this model, compartment mixing occurs on cortical granule-plasma membrane fusion and is required for actin assembly. Compartment mixing triggers actin assembly, at least in part, through diacylglycerol (DAG), which incorporates into the cortical granule membranes from the plasma membrane after cortical granule-plasma membrane fusion. DAG, in turn, directs long-term recruitment of protein kinase Cbeta (PKCbeta) to exocytosing cortical granules, where it is required for activation of Cdc42 localized on the cortical granules. The results demonstrate that mixing of two membrane compartments can direct local actin assembly and indicate that this process is harnessed during Xenopus egg cortical granule exocytosis to drive compensatory endocytosis.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures




Similar articles
-
Multiple myosins are required to coordinate actin assembly with coat compression during compensatory endocytosis.Mol Biol Cell. 2007 Oct;18(10):4096-105. doi: 10.1091/mbc.e06-11-0993. Epub 2007 Aug 15. Mol Biol Cell. 2007. PMID: 17699600 Free PMC article.
-
Kiss-and-coat and compartment mixing: coupling exocytosis to signal generation and local actin assembly.Mol Biol Cell. 2006 Apr;17(4):1495-502. doi: 10.1091/mbc.e05-10-0908. Epub 2006 Jan 25. Mol Biol Cell. 2006. PMID: 16436510 Free PMC article. Review.
-
Cdc42-dependent actin polymerization during compensatory endocytosis in Xenopus eggs.Nat Cell Biol. 2003 Aug;5(8):727-32. doi: 10.1038/ncb1025. Nat Cell Biol. 2003. PMID: 12872130
-
Evidence that the ability to respond to a calcium stimulus in exocytosis is determined by the secretory granule membrane: comparison of exocytosis of injected bovine chromaffin granule membranes and endogenous cortical granules in Xenopus laevis oocytes.Cell Mol Neurobiol. 1994 Jun;14(3):245-57. doi: 10.1007/BF02088323. Cell Mol Neurobiol. 1994. PMID: 7712514 Free PMC article.
-
Role of actin in regulated exocytosis and compensatory membrane retrieval: insights from an old acquaintance.Biochem Biophys Res Commun. 1999 Dec 29;266(3):652-61. doi: 10.1006/bbrc.1999.1883. Biochem Biophys Res Commun. 1999. PMID: 10603303 Review.
Cited by
-
Reciprocal regulation of AKT and MAP kinase dictates virus-host cell fusion.J Virol. 2010 May;84(9):4366-82. doi: 10.1128/JVI.01940-09. Epub 2010 Feb 17. J Virol. 2010. PMID: 20164223 Free PMC article.
-
Arp2/3-mediated F-actin formation controls regulated exocytosis in vivo.Nat Commun. 2015 Dec 7;6:10098. doi: 10.1038/ncomms10098. Nat Commun. 2015. PMID: 26639106 Free PMC article.
-
A localized calcium transient and polar body abscission.Cell Cycle. 2022 Nov;21(21):2239-2254. doi: 10.1080/15384101.2022.2092815. Epub 2022 Jul 1. Cell Cycle. 2022. PMID: 35775922 Free PMC article.
-
Regulation of fusion pore closure and compound exocytosis in neuroendocrine PC12 cells by SCAMP1.Traffic. 2011 May;12(5):600-14. doi: 10.1111/j.1600-0854.2011.01170.x. Epub 2011 Feb 25. Traffic. 2011. PMID: 21272170 Free PMC article.
-
Actin depolymerisation and crosslinking join forces with myosin II to contract actin coats on fused secretory vesicles.J Cell Sci. 2015 Mar 15;128(6):1193-203. doi: 10.1242/jcs.165571. Epub 2015 Jan 30. J Cell Sci. 2015. PMID: 25637593 Free PMC article.
References
-
- May RC, Machesky LM. Phagocytosis and the actin cytoskeleton. J Cell Sci. 2001;114:1061–1077. - PubMed
-
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. - PubMed
-
- Bretscher MS, Aguado-Velasco C. Membrane traffic during cell locomotion. Curr Opin Cell Biol. 1998;10:537–541. - PubMed
-
- Sokac AM, Co C, Taunton J, Bement W. Cdc42-dependent actin polymerization during compensatory endocytosis in Xenopus eggs. Nature Cell Biol. 2003;5:727–732. - PubMed
-
- Nemoto T, Kojima T, Oshima A, Bito H, Kasai H. Stabilization of exocytosis by dynamic F-actin coating of zymogen granules in pancreatic acini. J Biol Chem. 2004;279:37544–37550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

