Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-neuroligin
- PMID: 17237775
- PMCID: PMC4755312
- DOI: 10.1038/nn1837
Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-neuroligin
Erratum in
- Nat Neurosci. 2008 Feb;11(2):238
Abstract
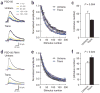
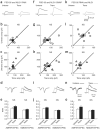
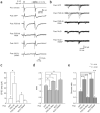
The structure and function of presynaptic and postsynaptic components of the synapse are highly coordinated. How such coordination is achieved and the molecules involved in this process have not been clarified. Several lines of evidence suggest that presynaptic functionalities are regulated by retrograde mechanisms from the postsynaptic side. We therefore sought postsynaptic mechanisms responsible for trans-synaptic regulation of presynaptic function at excitatory synapses in rat hippocampal CA1 pyramidal neurons. We show here that the postsynaptic complex of scaffolding protein PSD-95 and neuroligin can modulate the release probability of transmitter vesicles at synapse in a retrograde way, resulting in altered presynaptic short-term plasticity. Presynaptic beta-neurexin serves as a likely presynaptic mediator of this effect. Our results indicate that trans-synaptic protein-protein interactions can link postsynaptic and presynaptic function.
Figures




References
-
- Liu G, Choi S, Tsien RW. Variability of neurotransmitter concentration and nonsaturation of postsynaptic AMPA receptors at synapses in hippocampal cultures and slices. Neuron. 1999;22:395–409. - PubMed
-
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. - PubMed
-
- Rosenmund C, Clements JD, Westbrook GL. Nonuniform probability of glutamate release at a hippocampal synapse. Science. 1993;262:754–757. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

