Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states
- PMID: 17237794
- PMCID: PMC3138130
- DOI: 10.1038/nm1536
Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states
Erratum in
- Nat Med. 2007 Apr;13(4):511
Abstract
Skeletal muscle has the ability to achieve rapid repair in response to injury or disease. Many individuals with Marfan syndrome (MFS), caused by a deficiency of extracellular fibrillin-1, exhibit myopathy and often are unable to increase muscle mass despite physical exercise. Evidence suggests that selected manifestations of MFS reflect excessive signaling by transforming growth factor (TGF)-beta (refs. 2,3). TGF-beta is a known inhibitor of terminal differentiation of cultured myoblasts; however, the functional contribution of TGF-beta signaling to disease pathogenesis in various inherited myopathic states in vivo remains unknown. Here we show that increased TGF-beta activity leads to failed muscle regeneration in fibrillin-1-deficient mice. Systemic antagonism of TGF-beta through administration of TGF-beta-neutralizing antibody or the angiotensin II type 1 receptor blocker losartan normalizes muscle architecture, repair and function in vivo. Moreover, we show TGF-beta-induced failure of muscle regeneration and a similar therapeutic response in a dystrophin-deficient mouse model of Duchenne muscular dystrophy.
Conflict of interest statement
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
Figures
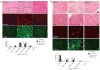
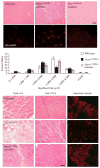
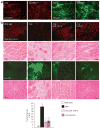

Comment in
-
ACE inhibitor bulks up muscle.Nat Med. 2007 Feb;13(2):125-6. doi: 10.1038/nm0207-125. Nat Med. 2007. PMID: 17290265 No abstract available.
References
-
- Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. - PubMed
-
- Neptune ER, et al. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–411. - PubMed
-
- Allen RE, Boxhorn LK. Inhibition of skeletal muscle satellite cell differentiation by transforming growth factor-beta. J Cell Physiol. 1987;133:567–572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

