Quantitative membrane proteomics reveals new cellular targets of viral immune modulators
- PMID: 17238276
- PMCID: PMC1626102
- DOI: 10.1371/journal.ppat.0020107
Quantitative membrane proteomics reveals new cellular targets of viral immune modulators
Abstract
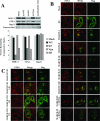
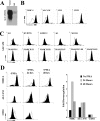
Immunomodulators of pathogens frequently affect multiple cellular targets, thus preventing recognition by different immune cells. For instance, the K5 modulator of immune recognition (MIR2) from Kaposi sarcoma-associated herpesvirus prevents activation of cytotoxic T cells, natural killer cells, and natural killer T cells by downregulating major histocompatibility complex (MHC) class I molecules, the MHC-like molecule CD1, the cell adhesion molecules ICAM-1 and PECAM, and the co-stimulatory molecule B7.2. K5 belongs to a family of viral- and cellular-membrane-spanning RING ubiquitin ligases. While a limited number of transmembrane proteins have been shown to be targeted for degradation by this family, it is unknown whether additional targets exist. We now describe a quantitative proteomics approach to identify novel targets of this protein family. Using stable isotope labeling by amino acids, we compared the proteome of plasma, Golgi, and endoplasmic reticulum membranes in the presence and absence of K5. Mass spectrometric protein identification revealed four proteins that were consistently underrepresented in the plasma membrane of K5 expression cells: MHC I (as expected), bone marrow stromal antigen 2 (BST-2, CD316), activated leukocyte cell adhesion molecule (ALCAM, CD166) and Syntaxin-4. Downregulation of each of these proteins was independently confirmed by immunoblotting with specific antibodies. We further demonstrate that ALCAM is a bona fide target of both K5 and the myxomavirus homolog M153R. Upon exiting the endoplasmic reticulum, ALCAM is ubiquitinated in the presence of wild-type, but not RING-deficient or acidic motif-deficient, K5, and is targeted for lysosomal degradation via the multivesicular body pathway. Since ALCAM is the ligand for CD6, a member of the immunological synapse of T cells, its removal by viral immune modulators implies a role for CD6 in the recognition of pathogens by T cells. The unbiased global proteome analysis therefore revealed novel immunomodulatory functions of pathogen proteins.
Conflict of interest statement
Figures




References
-
- Wiertz EJ, Jones TR, Sun L, Bogyo M, Geuze HJ, et al. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell. 1996;84:769–779. - PubMed
-
- Johnson DC, Hegde NR. Inhibition of the MHC class II antigen presentation pathway by human cytomegalovirus. Curr Top Microbiol Immunol. 2002;269:101–115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

