Fgf-dependent otic induction requires competence provided by Foxi1 and Dlx3b
- PMID: 17239227
- PMCID: PMC1794237
- DOI: 10.1186/1471-213X-7-5
Fgf-dependent otic induction requires competence provided by Foxi1 and Dlx3b
Abstract
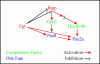
Background: The inner ear arises from a specialized set of cells, the otic placode, that forms at the lateral edge of the neural plate adjacent to the hindbrain. Previous studies indicated that fibroblast growth factors (Fgfs) are required for otic induction; in zebrafish, loss of both Fgf3 and Fgf8 results in total ablation of otic tissue. Furthermore, gain-of-function studies suggested that Fgf signaling is not only necessary but also sufficient for otic induction, although the amount of induced ectopic otic tissue reported after misexpression of fgf3 or fgf8 varies among different studies. We previously suggested that Foxi1 and Dlx3b may provide competence to form the ear because loss of both foxi1 and dlx3b results in ablation of all otic tissue even in the presence of a fully functional Fgf signaling pathway.
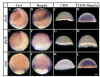
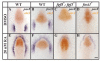
Results: Using a transgenic line that allows us to misexpress fgf8 under the control of the zebrafish temperature-inducible hsp70 promoter, we readdressed the role of Fgf signaling and otic competence during placode induction. We find that misexpression of fgf8 fails to induce formation of ectopic otic vesicles outside of the endogenous ear field and has different consequences depending upon the developmental stage. Overexpression of fgf8 from 1-cell to midgastrula stages leads to formation of no or small otic vesicles, respectively. Overexpression of fgf8 at these stages never leads to ectopic expression of foxi1 or dlx3b, contrary to previous studies that indicated that foxi1 is activated by Fgf signaling. Consistent with our results we find that pharmacological inhibition of Fgf signaling has no effect on foxi1 or dlx3b expression, but instead, Bmp signaling activates foxi1, directly and dlx3b, indirectly. In contrast to early activation of fgf8, fgf8 overexpression at the end of gastrulation, when otic induction begins, leads to much larger otic vesicles. We further show that application of a low dose of retinoic acid that does not perturb patterning of the anterior neural plate leads to expansion of foxi1 and to a massive Fgf-dependent otic induction.
Conclusion: These results provide further support for the hypothesis that Foxi1 and Dlx3b provide competence for cells to respond to Fgf and form an otic placode.
Figures






Similar articles
-
Genetic interactions underlying otic placode induction and formation.Dev Dyn. 2004 Jul;230(3):419-33. doi: 10.1002/dvdy.20067. Dev Dyn. 2004. PMID: 15188428
-
Fgf8 and Fgf3 are required for zebrafish ear placode induction, maintenance and inner ear patterning.Mech Dev. 2002 Nov;119(1):91-108. doi: 10.1016/s0925-4773(02)00343-x. Mech Dev. 2002. PMID: 12385757
-
Zebrafish Foxi1 provides a neuronal ground state during inner ear induction preceding the Dlx3b/4b-regulated sensory lineage.Development. 2013 May;140(9):1936-45. doi: 10.1242/dev.087718. Development. 2013. PMID: 23571216
-
Expression and functions of FGF ligands during early otic development.Int J Dev Biol. 2007;51(6-7):473-81. doi: 10.1387/ijdb.072334ts. Int J Dev Biol. 2007. PMID: 17891710 Review.
-
Determination of the embryonic inner ear.J Neurobiol. 2002 Nov 5;53(2):100-28. doi: 10.1002/neu.10131. J Neurobiol. 2002. PMID: 12382270 Review.
Cited by
-
Fgf3 and Fgf10a work in concert to promote maturation of the epibranchial placodes in zebrafish.PLoS One. 2013 Dec 17;8(12):e85087. doi: 10.1371/journal.pone.0085087. eCollection 2013. PLoS One. 2013. PMID: 24358375 Free PMC article.
-
Zebrafish Tbx16 regulates intermediate mesoderm cell fate by attenuating Fgf activity.Dev Biol. 2013 Nov 1;383(1):75-89. doi: 10.1016/j.ydbio.2013.08.018. Epub 2013 Sep 2. Dev Biol. 2013. PMID: 24008197 Free PMC article.
-
Fgf signaling governs cell fate in the zebrafish pineal complex.Development. 2013 Jan 15;140(2):323-32. doi: 10.1242/dev.083709. Development. 2013. PMID: 23250206 Free PMC article.
-
Conditions that influence the response to Fgf during otic placode induction.Dev Biol. 2012 Apr 1;364(1):1-10. doi: 10.1016/j.ydbio.2012.01.022. Epub 2012 Feb 1. Dev Biol. 2012. PMID: 22327005 Free PMC article.
-
Foxi3 is necessary for the induction of the chick otic placode in response to FGF signaling.Dev Biol. 2014 Jul 15;391(2):158-69. doi: 10.1016/j.ydbio.2014.04.014. Epub 2014 Apr 26. Dev Biol. 2014. PMID: 24780628 Free PMC article.
References
-
- Noden DM, van de Water TR. The developing ear: tissue orgins and interactions. In: Ruben RJ,van de Water TR, Rubel EW, editor. The Biology of Change in Otolaryngology. Amsterdam: Elsevier; 1986. pp. 15–46.
-
- Couly GF, Coltey PM, Le Douarin NM. The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development. 1993;117:409–429. - PubMed
-
- Fritzsch BF, Barald KF, Lomax MI. Early embryology of the vertebrate ear. In: Rubel EW, Popper AN, Fay RR, editor. Development of the Auditory System. New York Springer Verlag; 1997. pp. 80–145.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

