Temperature dependence of erythromelalgia mutation L858F in sodium channel Nav1.7
- PMID: 17239250
- PMCID: PMC1781932
- DOI: 10.1186/1744-8069-3-3
Temperature dependence of erythromelalgia mutation L858F in sodium channel Nav1.7
Abstract
Background: The disabling chronic pain syndrome erythromelalgia (also termed erythermalgia) is characterized by attacks of burning pain in the extremities induced by warmth. Pharmacological treatment is often ineffective, but the pain can be alleviated by cooling of the limbs. Inherited erythromelalgia has recently been linked to mutations in the gene SCN9A, which encodes the voltage-gated sodium channel Nav1.7. Nav1.7 is preferentially expressed in most nociceptive DRG neurons and in sympathetic ganglion neurons. It has recently been shown that several disease-causing erythromelalgia mutations alter channel-gating behavior in a manner that increases DRG neuron excitability.
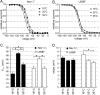
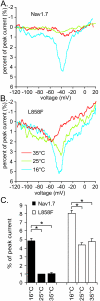
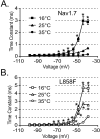
Results: Here we tested the effects of temperature on gating properties of wild type Nav1.7 and mutant L858F channels. Whole-cell voltage-clamp measurements on wild type or L858F channels expressed in HEK293 cells revealed that cooling decreases current density, slows deactivation and increases ramp currents for both mutant and wild type channels. However, cooling differentially shifts the midpoint of steady-state activation in a depolarizing direction for L858F but not for wild type channels.
Conclusion: The cooling-dependent shift of the activation midpoint of L858F to more positive potentials brings the threshold of activation of the mutant channels closer to that of wild type Nav1.7 at lower temperatures, and is likely to contribute to the alleviation of painful symptoms upon cooling in affected limbs in patients with this erythromelalgia mutation.
Figures


Similar articles
-
Deletion mutation of sodium channel Na(V)1.7 in inherited erythromelalgia: enhanced slow inactivation modulates dorsal root ganglion neuron hyperexcitability.Brain. 2011 Jul;134(Pt 7):1972-86. doi: 10.1093/brain/awr143. Brain. 2011. PMID: 21705421
-
Sporadic onset of erythermalgia: a gain-of-function mutation in Nav1.7.Ann Neurol. 2006 Mar;59(3):553-8. doi: 10.1002/ana.20776. Ann Neurol. 2006. PMID: 16392115
-
Erythromelalgia mutation L823R shifts activation and inactivation of threshold sodium channel Nav1.7 to hyperpolarized potentials.Biochem Biophys Res Commun. 2009 Dec 11;390(2):319-24. doi: 10.1016/j.bbrc.2009.09.121. Epub 2009 Oct 1. Biochem Biophys Res Commun. 2009. PMID: 19800314
-
[From gene to disease; primary erythermalgia--a neuropathic disease as a consequence of mutations in a sodium pump gene].Ned Tijdschr Geneeskd. 2006 Jan 28;150(4):194-6. Ned Tijdschr Geneeskd. 2006. PMID: 16471234 Review. Dutch.
-
Erythermalgia: molecular basis for an inherited pain syndrome.Trends Mol Med. 2005 Dec;11(12):555-62. doi: 10.1016/j.molmed.2005.10.004. Epub 2005 Nov 8. Trends Mol Med. 2005. PMID: 16278094 Review.
Cited by
-
Erythromelalgia: a cutaneous manifestation of neuropathy?An Bras Dermatol. 2018 Jan-Feb;93(1):86-94. doi: 10.1590/abd1806-4841.20187535. An Bras Dermatol. 2018. PMID: 29641704 Free PMC article. Review.
-
Febrile temperatures unmask biophysical defects in Nav1.1 epilepsy mutations supportive of seizure initiation.J Gen Physiol. 2013 Dec;142(6):641-53. doi: 10.1085/jgp.201311042. J Gen Physiol. 2013. PMID: 24277604 Free PMC article.
-
A novel SCN9A mutation responsible for primary erythromelalgia and is resistant to the treatment of sodium channel blockers.PLoS One. 2013;8(1):e55212. doi: 10.1371/journal.pone.0055212. Epub 2013 Jan 31. PLoS One. 2013. PMID: 23383113 Free PMC article.
-
Anomalous enhancement of resurgent Na+ currents at high temperatures by SCN9A mutations underlies the episodic heat-enhanced pain in inherited erythromelalgia.Sci Rep. 2019 Aug 22;9(1):12251. doi: 10.1038/s41598-019-48672-6. Sci Rep. 2019. PMID: 31439884 Free PMC article.
-
Mutations in sodium-channel gene SCN9A cause a spectrum of human genetic pain disorders.J Clin Invest. 2007 Dec;117(12):3603-9. doi: 10.1172/JCI33297. J Clin Invest. 2007. PMID: 18060017 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

