phospholipase C, beta 3 is required for Endothelin1 regulation of pharyngeal arch patterning in zebrafish
- PMID: 17239364
- PMCID: PMC1906931
- DOI: 10.1016/j.ydbio.2006.12.027
phospholipase C, beta 3 is required for Endothelin1 regulation of pharyngeal arch patterning in zebrafish
Abstract
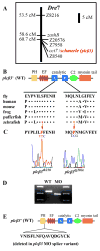
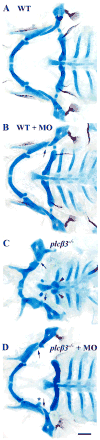
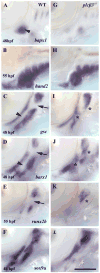
Genetic and pharmacological studies demonstrate that Endothelin1 (Edn1) is a key signaling molecule for patterning the facial skeleton in fish, chicks, and mice. When Edn1 function is reduced early in development the ventral lower jaw and supporting structures are reduced in size and often fused to their dorsal upper jaw counterparts. We show that schmerle (she) encodes a zebrafish ortholog of Phospholipase C, beta 3 (Plcbeta3) required in cranial neural crest cells for Edn1 regulation of pharyngeal arch patterning. Sequencing and co-segregation demonstrates that two independent she (plcbeta3) alleles have missense mutations in conserved residues within the catalytic domains of Plcbeta3. Homozygous plcbeta3 mutants are phenotypically similar to edn1 mutants and exhibit a strong arch expression defect in Edn1-dependent Distalless (Dlx) genes as well as expression defects in several Edn1-dependent intermediate and ventral arch domain transcription factors. plcbeta3 also genetically interacts with edn1, supporting a model in which Edn1 signals through a G protein-coupled receptor to activate Plcbeta3. Mild skeletal defects occur in plcbeta3 heterozygotes, showing the plcbeta3 mutations are partially dominant. Through a morpholino-mediated deletion in the N-terminal PH domain of Plcbeta3, we observe a partial rescue of facial skeletal defects in homozygous plcbeta3 mutants, supporting a hypothesis that an intact PH domain is necessary for the partial dominance we observe. In addition, through mosaic analyses, we show that wild-type neural crest cells can efficiently rescue facial skeletal defects in homozygous plcbeta3 mutants, demonstrating that Plcbeta3 function is required in neural crest cells and not other cell types to pattern the facial skeleton.
Figures






Similar articles
-
mef2ca is required in cranial neural crest to effect Endothelin1 signaling in zebrafish.Dev Biol. 2007 Aug 1;308(1):144-57. doi: 10.1016/j.ydbio.2007.05.018. Epub 2007 May 24. Dev Biol. 2007. PMID: 17574232 Free PMC article.
-
Zebrafish furin mutants reveal intricacies in regulating Endothelin1 signaling in craniofacial patterning.Dev Biol. 2006 Jul 1;295(1):194-205. doi: 10.1016/j.ydbio.2006.03.028. Epub 2006 Mar 30. Dev Biol. 2006. PMID: 16678149
-
Requirements for Endothelin type-A receptors and Endothelin-1 signaling in the facial ectoderm for the patterning of skeletogenic neural crest cells in zebrafish.Development. 2007 Jan;134(2):335-45. doi: 10.1242/dev.02704. Epub 2006 Dec 13. Development. 2007. PMID: 17166927
-
Regulation of facial morphogenesis by endothelin signaling: insights from mice and fish.Am J Med Genet A. 2010 Dec;152A(12):2962-73. doi: 10.1002/ajmg.a.33568. Am J Med Genet A. 2010. PMID: 20684004 Free PMC article. Review.
-
Understanding endothelin-1 function during craniofacial development in the mouse and zebrafish.Birth Defects Res C Embryo Today. 2004 Jun;72(2):190-9. doi: 10.1002/bdrc.20007. Birth Defects Res C Embryo Today. 2004. PMID: 15269892 Review.
Cited by
-
Competition between Jagged-Notch and Endothelin1 Signaling Selectively Restricts Cartilage Formation in the Zebrafish Upper Face.PLoS Genet. 2016 Apr 8;12(4):e1005967. doi: 10.1371/journal.pgen.1005967. eCollection 2016 Apr. PLoS Genet. 2016. PMID: 27058748 Free PMC article.
-
The Gq/11 family of Gα subunits is necessary and sufficient for lower jaw development.Development. 2025 Apr 15;152(8):dev204396. doi: 10.1242/dev.204396. Epub 2025 Apr 17. Development. 2025. PMID: 40171762 Free PMC article.
-
MicroRNAs and micromanaging the skeleton in disease, development and evolution.J Cell Mol Med. 2009 Apr;13(4):606-18. doi: 10.1111/j.1582-4934.2009.00696.x. Epub 2009 Feb 9. J Cell Mol Med. 2009. PMID: 19220576 Free PMC article. Review.
-
Transgenic line with gal4 insertion useful to study morphogenesis of craniofacial perichondrium, vascular endothelium-associated cells, floor plate, and dorsal midline radial glia during zebrafish development.Dev Growth Differ. 2012 Feb;54(2):202-15. doi: 10.1111/j.1440-169X.2011.01322.x. Epub 2012 Feb 20. Dev Growth Differ. 2012. PMID: 22348745 Free PMC article.
-
Knockdown of hspg2 is associated with abnormal mandibular joint formation and neural crest cell dysfunction in zebrafish.BMC Dev Biol. 2021 Mar 8;21(1):7. doi: 10.1186/s12861-021-00238-4. BMC Dev Biol. 2021. PMID: 33678174 Free PMC article.
References
-
- Appel B, Korzh V, Glasgow E, Thor S, Edlund T, Dawid IB, Eisen JS. Motoneuron fate specification revealed by patterned LIM homeobox gene expression in embryonic zebrafish. Development. 1995;121:4117–25. - PubMed
-
- Clouthier DE, Hosoda K, Richardson JA, Williams SC, Yanagisawa H, Kuwaki T, Kumada M, Hammer RE, Yanagisawa M. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development. 1998;125:813–24. - PubMed
-
- Clouthier DE, Williams SC, Hammer RE, Richardson JA, Yanagisawa M. Cell-autonomous and nonautonomous actions of endothelin-A receptor signaling in craniofacial and cardiovascular development. Dev Biol. 2003;261:506–19. - PubMed
-
- Clouthier DE, Williams SC, Yanagisawa H, Wieduwilt M, Richardson JA, Yanagisawa M. Signaling pathways crucial for craniofacial development revealed by endothelin-A receptor-deficient mice. Dev Biol. 2000;217:10–24. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

