Storage of an oculomotor motion aftereffect
- PMID: 17239421
- PMCID: PMC2564621
- DOI: 10.1016/j.visres.2006.09.030
Storage of an oculomotor motion aftereffect
Abstract
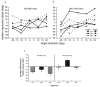
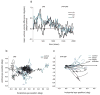
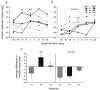
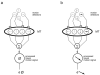
Adaptation to motion produces a motion aftereffect (MAE), where illusory, oppositely-directed motion is perceived when viewing a stationary image. A common hypothesis for motion adaptation is that it reflects an imbalance of activity caused by neuronal fatigue. However, the perceptual MAE exhibits storage, in that the MAE appears even after a prolonged period of darkness is interposed between the adapting stimulus and the test, suggesting that fatigue cannot explain the perceptual MAE. We asked whether neural fatigue was a viable explanation for the oculomotor MAE (OMAE) by testing if the OMAE exhibits storage. Human observers were adapted with moving, random-dot cinematograms. Following adaptation, they generated an oculomotor MAE (OMAE), with both pursuit and saccadic components. The OMAE occurred in the presence of a visual test stimulus, but not in the dark. When the test stimulus was introduced after the dark period, the OMAE reappeared, analogous to perceptual MAE storage. The results suggest that fatigue cannot explain the OMAE, and that visual stimulation is necessary to elicit it. We propose a model in which adaptation recalibrates the motion-processing network by adjusting the weights of the inputs to neurons in the middle-temporal (MT) area.
Conflict of interest statement
Competing interests statement
The authors declare that they have no competing financial interests.
Figures


Similar articles
-
Eye movements and the motion aftereffect: alternatives to the induced motion hypothesis.Vision Res. 1991;31(9):1639-45. doi: 10.1016/0042-6989(91)90141-q. Vision Res. 1991. PMID: 1949633
-
Extra-retinal adaptation of cortical motion-processing areas during pursuit eye movements.Proc Biol Sci. 2005 Oct 22;272(1577):2127-32. doi: 10.1098/rspb.2005.3198. Proc Biol Sci. 2005. PMID: 16191625 Free PMC article.
-
Motion aftereffect of combined first-order and second-order motion.Perception. 1999;28(11):1397-411. doi: 10.1068/p2899. Perception. 1999. PMID: 10755148
-
Eye movements and perception: a selective review.J Vis. 2011 Sep 14;11(5):9. doi: 10.1167/11.5.9. J Vis. 2011. PMID: 21917784 Review.
-
And yet it moves: perceptual illusions and neural mechanisms of pursuit compensation during smooth pursuit eye movements.Neurosci Biobehav Rev. 2012 Jan;36(1):143-51. doi: 10.1016/j.neubiorev.2011.05.005. Epub 2011 May 17. Neurosci Biobehav Rev. 2012. PMID: 21616092 Review.
Cited by
-
Emotional Actions Are Coded via Two Mechanisms: With and without Identity Representation.Front Psychol. 2016 May 11;7:693. doi: 10.3389/fpsyg.2016.00693. eCollection 2016. Front Psychol. 2016. PMID: 27242606 Free PMC article.
-
The influence of motion signals in hand movements.Exp Brain Res. 2008 Nov;191(3):321-9. doi: 10.1007/s00221-008-1527-1. Epub 2008 Aug 14. Exp Brain Res. 2008. PMID: 18704383
-
Afternystagmus in darkness after suppression of optokinetic nystagmus: an interaction of motion aftereffect and retinal afterimages.Exp Brain Res. 2014 Sep;232(9):2891-8. doi: 10.1007/s00221-014-3971-4. Epub 2014 May 13. Exp Brain Res. 2014. PMID: 24820290 Clinical Trial.
-
Sustained Rhythmic Brain Activity Underlies Visual Motion Perception in Zebrafish.Cell Rep. 2016 Oct 18;17(4):1098-1112. doi: 10.1016/j.celrep.2016.09.065. Cell Rep. 2016. PMID: 27760314 Free PMC article.
-
The effects of prolonged viewing of motion on short-latency ocular following responses.Exp Brain Res. 2009 May;195(2):195-205. doi: 10.1007/s00221-009-1768-7. Epub 2009 Mar 24. Exp Brain Res. 2009. PMID: 19308363
References
-
- Addams RA. An account of a peculiar optical phenomenon seen after having looked at a moving body. London and Edinburgh Philosophical Magazine and Journal of Science. 1834;5:373–374.
-
- Andrews DP. Error-correcting perceptual mechanisms. Q J Exp Psychol. 1964;16:104–115.
-
- Ball K, Sekuler R. Masking of motion by broadband and filtered directional noise. Perception & Psychophysics. 1979;26:206–214.
-
- Barlow HB, Földiåk P. Adaptation and decorrelation in the cortex. In: Durbin R, Miall C, Mitchison G, editors. The Computing Neuron. 1989. pp. 54–72.
-
- Barlow HB, Hill RM. Evidence for a physiological explanation for the waterfall phenomenon and figural aftereffects. Nature. 1963;200:1345–1347. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

