A papillomavirus-like particle (VLP) vaccine displaying HPV16 L2 epitopes induces cross-neutralizing antibodies to HPV11
- PMID: 17239496
- PMCID: PMC3935451
- DOI: 10.1016/j.vaccine.2006.11.049
A papillomavirus-like particle (VLP) vaccine displaying HPV16 L2 epitopes induces cross-neutralizing antibodies to HPV11
Abstract
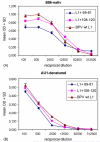
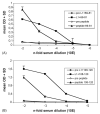
Peptides of the papillomavirus L2 minor capsid protein can induce antibodies (Ab) that neutralize a broad range of human papillomavirus (HPV) genotypes. Unfortunately, L2 is antigenically subdominant to L1 in the virus capsid. To induce a strong anti-L2 Ab response with cross-neutralizing activity to other mucosal types, chimeric virus-like particles (VLP) were generated in which HPV16 L2 neutralization epitopes (comprising L2 residues 69-81 or 108-120) are inserted within an immunodominant surface loop (between residues 133 and 134) of the L1 major capsid protein of bovine papillomavirus type 1 (BPV1). These chimeras self-assembled into pentameric capsomers, or complete VLP similar to wild type (wt) L1 protein. Immunization of rabbits with assembled particle preparations induced L2-specific serum Ab with titers 10-fold higher than those induced by cognate synthetic L2 peptides coupled to KLH. Antisera to both chimeric proteins partially neutralized HPV16 pseudovirions, confirming that both HPV16 L2 peptides define neutralization epitopes. When analyzed for the ability to cross-neutralize infection by authentic HPV11 virions, using detection of early viral RNA by RT-PCR-assays as the readout, immune serum to chimeric protein comprising L2 residues 69-81, but not 108-120, was partially neutralizing. In addition, mouse-antiserum induced by vaccinations with synthetic L2 peptide 108-120, but not 69-81, was partially neutralizing in this assay. Induction of cross-neutralization Ab by L2 epitopes displayed on chimeric VLP represents a possible strategy for the generation of broad-spectrum vaccines to protect against relevant mucosal HPV and associated neoplasia.
Figures





Similar articles
-
Neutralization of HPV16, 18, 31, and 58 pseudovirions with antisera induced by immunizing rabbits with synthetic peptides representing segments of the HPV16 minor capsid protein L2 surface region.Virology. 2007 Feb 20;358(2):266-72. doi: 10.1016/j.virol.2006.08.037. Epub 2006 Sep 28. Virology. 2007. PMID: 17010405
-
Chimeric L1-L2 virus-like particles as potential broad-spectrum human papillomavirus vaccines.J Virol. 2009 Oct;83(19):10085-95. doi: 10.1128/JVI.01088-09. Epub 2009 Jul 29. J Virol. 2009. PMID: 19640991 Free PMC article.
-
Broad Cross-Protection Is Induced in Preclinical Models by a Human Papillomavirus Vaccine Composed of L1/L2 Chimeric Virus-Like Particles.J Virol. 2016 Jun 24;90(14):6314-25. doi: 10.1128/JVI.00449-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27147749 Free PMC article.
-
Developments in L2-based human papillomavirus (HPV) vaccines.Virus Res. 2017 Mar 2;231:166-175. doi: 10.1016/j.virusres.2016.11.020. Epub 2016 Nov 23. Virus Res. 2017. PMID: 27889616 Free PMC article. Review.
-
Chapter 12: Prophylactic HPV vaccines: underlying mechanisms.Vaccine. 2006 Aug 31;24 Suppl 3:S3/106-13. doi: 10.1016/j.vaccine.2006.05.110. Epub 2006 Jun 23. Vaccine. 2006. PMID: 16949996 Review.
Cited by
-
A Survey of Preclinical Studies Evaluating Nanoparticle-Based Vaccines Against Non-Viral Sexually Transmitted Infections.Front Pharmacol. 2021 Nov 24;12:768461. doi: 10.3389/fphar.2021.768461. eCollection 2021. Front Pharmacol. 2021. PMID: 34899322 Free PMC article. Review.
-
HPV entry into cells.Mutat Res Rev Mutat Res. 2017 Apr-Jun;772:13-22. doi: 10.1016/j.mrrev.2016.09.004. Epub 2016 Sep 23. Mutat Res Rev Mutat Res. 2017. PMID: 28528686 Free PMC article. Review.
-
Virus-like particle vaccine displaying an external, membrane adjacent MUC16 epitope elicits ovarian cancer-reactive antibodies.J Ovarian Res. 2024 Jan 16;17(1):19. doi: 10.1186/s13048-023-01325-9. J Ovarian Res. 2024. PMID: 38225646 Free PMC article.
-
Papillomavirus prophylactic vaccines: established successes, new approaches.J Virol. 2010 Feb;84(3):1214-20. doi: 10.1128/JVI.01927-09. Epub 2009 Nov 11. J Virol. 2010. PMID: 19906917 Free PMC article. Review.
-
[HPV vaccines. Prophylactic vaccines from virus-like particles].Hautarzt. 2007 Jun;58(6):489-92. doi: 10.1007/s00105-007-1341-x. Hautarzt. 2007. PMID: 17483914 Review. German.
References
-
- de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. - PubMed
-
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. - PubMed
-
- Pfister H. Chapter 8: human papillomavirus and skin cancer. J Natl Cancer Inst Monogr. 2003:52–6. - PubMed
-
- Salzman NP, Howley PM, editors. The Papillomaviruses. Vol. 2. Plenum Press; New York: 1987. The Papovaviridae; p. 392.
-
- zur Hausen H. Papillomavirus infection- a major cause of human cancers. Biochim Biophys Acta. 1996;1288:F55–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

