Regulation of carnitine palmitoyltransferase I (CPT-Ialpha) gene expression by the peroxisome proliferator activated receptor gamma coactivator (PGC-1) isoforms
- PMID: 17239528
- PMCID: PMC1892282
- DOI: 10.1016/j.mce.2006.11.012
Regulation of carnitine palmitoyltransferase I (CPT-Ialpha) gene expression by the peroxisome proliferator activated receptor gamma coactivator (PGC-1) isoforms
Abstract
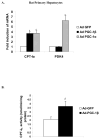
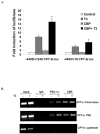
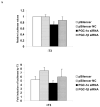
The peroxisome proliferator activated receptor gamma coactivators (PGC-1) have important roles in mitochondrial biogenesis and metabolic control in a variety of tissues. There are multiple isoforms of PGC-1 including PGC-1alpha and PGC-1beta. Both the PGC-1alpha and beta isoforms promote mitochondrial biogenesis and fatty acid oxidation, but only PGC-1alpha stimulates gluconeogenesis in the liver. Carnitine palmitoyltransferase I (CPT-I) is a key enzyme regulating mitochondrial fatty acid oxidation. In these studies, we determined that PGC-1beta stimulated expression of the "liver" isoform of CPT-I (CPT-Ialpha) but that PGC-1beta did not induce pyruvate dehydrogenase kinase 4 (PDK4) which is a regulator of pyruvate metabolism. The CPT-Ialpha gene is induced by thyroid hormone. We found that T3 increased the expression of PGC-1beta and that PGC-1beta enhanced the T3 induction of CPT-Ialpha. The thyroid hormone receptor interacts with PGC-1beta in a ligand dependent manner. Unlike PGC-1alpha, the interaction of PGC-1beta and the T3 receptor does not occur exclusively through the leucine-X-X-leucine-leucine motif in PGC-1beta. We have found that PGC-1beta is associated with the CPT-Ialpha gene in vivo. Overall, our results demonstrate that PGC-1beta is a coactivator in the T3 induction of CPT-Ialpha and that PGC-1beta has similarities and differences with the PGC-1alpha isoform.
Figures

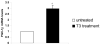







Similar articles
-
Peroxisomal proliferator activated receptor gamma coactivator (PGC-1alpha) stimulates carnitine palmitoyltransferase I (CPT-Ialpha) through the first intron.Biochim Biophys Acta. 2004 Aug 12;1679(2):164-73. doi: 10.1016/j.bbaexp.2004.06.006. Biochim Biophys Acta. 2004. PMID: 15297149
-
Peroxisomal proliferator-activated receptor-gamma coactivator-1 alpha (PGC-1 alpha) enhances the thyroid hormone induction of carnitine palmitoyltransferase I (CPT-I alpha).J Biol Chem. 2004 Dec 24;279(52):53963-71. doi: 10.1074/jbc.M406028200. Epub 2004 Oct 6. J Biol Chem. 2004. PMID: 15469941
-
Peroxisome proliferator activated receptor alpha (PPARalpha) and PPAR gamma coactivator (PGC-1alpha) induce carnitine palmitoyltransferase IA (CPT-1A) via independent gene elements.Mol Cell Endocrinol. 2010 Aug 30;325(1-2):54-63. doi: 10.1016/j.mce.2010.05.019. Mol Cell Endocrinol. 2010. PMID: 20638986 Free PMC article.
-
Expression and regulation of carnitine palmitoyltransferase-Ialpha and -Ibeta genes.Am J Med Sci. 1999 Jul;318(1):43-8. doi: 10.1097/00000441-199907000-00007. Am J Med Sci. 1999. PMID: 10408760 Review.
-
Regulation of liver carnitine palmitoyltransferase I gene expression by hormones and fatty acids.Biochem Soc Trans. 2001 May;29(Pt 2):310-6. doi: 10.1042/0300-5127:0290310. Biochem Soc Trans. 2001. PMID: 11356173 Review.
Cited by
-
Thyroid hormone analogues and derivatives: Actions in fatty liver.World J Hepatol. 2014 Mar 27;6(3):114-29. doi: 10.4254/wjh.v6.i3.114. World J Hepatol. 2014. PMID: 24672641 Free PMC article. Review.
-
Analysis of the heat shock response in mouse liver reveals transcriptional dependence on the nuclear receptor peroxisome proliferator-activated receptor alpha (PPARalpha).BMC Genomics. 2010 Jan 7;11:16. doi: 10.1186/1471-2164-11-16. BMC Genomics. 2010. PMID: 20059764 Free PMC article.
-
Metabolomic and Lipidomic Analysis of the Heart of Peroxisome Proliferator-Activated Receptor-γ Coactivator 1-β Knock Out Mice on a High Fat Diet.Metabolites. 2012 Jun 18;2(2):366-81. doi: 10.3390/metabo2020366. Metabolites. 2012. PMID: 24957515 Free PMC article.
-
The interplay of prolactin and the glucocorticoids in the regulation of beta-cell gene expression, fatty acid oxidation, and glucose-stimulated insulin secretion: implications for carbohydrate metabolism in pregnancy.Endocrinology. 2008 Nov;149(11):5401-14. doi: 10.1210/en.2008-0051. Epub 2008 Jul 3. Endocrinology. 2008. PMID: 18599550 Free PMC article.
-
PGC-1 coactivators in cardiac development and disease.Circ Res. 2010 Oct 1;107(7):825-38. doi: 10.1161/CIRCRESAHA.110.223818. Circ Res. 2010. PMID: 20884884 Free PMC article. Review.
References
-
- McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem. 1997;244:1–14. - PubMed
-
- Cook GA, Park EA. Expression and regulation of carnitine palmitoyltransferase-Ialpha and -Ibeta genes. Am J Med Sci. 1999;318:43–8. - PubMed
-
- Price N, van der Leij F, Jackson V, Corstorphine C, Thomson R, Sorensen A, Zammit V. A novel brain-expressed protein related to carnitine palmitoyltransferase I. Genomics. 2002;80:433–42. - PubMed
-
- Jansen MS, Cook GA, Song S, Park EA. Thyroid hormone regulates carnitine palmitoyltransferase Ialpha gene expression through elements in the promoter and first intron. J Biol Chem. 2000;275:34989–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

