Sequential induction of pro- and anti-inflammatory prostaglandins and peroxisome proliferators-activated receptor-gamma during normal wound healing: a time course study
- PMID: 17239574
- PMCID: PMC1847382
- DOI: 10.1016/j.plefa.2006.11.006
Sequential induction of pro- and anti-inflammatory prostaglandins and peroxisome proliferators-activated receptor-gamma during normal wound healing: a time course study
Abstract
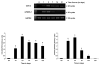
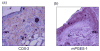
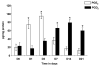
Lipid mediators generated from metabolism of arachidonic acid play a crucial role in the initiating and resolution of acute inflammation by shifting from pro-inflammatory prostaglandin (PG) E2 to anti-inflammatory PGD2 and its metabolites. The changes in PG levels over time during the normal wound-repair process have not, however, been reported. We determined the temporal expression of PG and their biosynthetic enzymes using the full thickness incisional model of normal wound healing in mice. We demonstrate that during normal wound repair, there is a shift in the metabolism of arachidonate from PGE2 during the acute inflammatory phase to PGD2 during the repair phase. This shift is mediated by temporal changes in the expression of cyclooxygenases (COX) and microsomal PGES (mPGES)-1. Inducible COX (COX-2) expression is sustained throughout the initiation and repair process, but mPGES-1 is increased only during the acute inflammatory phase and its disappearance coincides with increased PGD2. PGD2 and its degradation products are known to mediate their anti-inflammatory effects by binding to peroxisome proliferators-activated receptor gamma (PPARgamma). In this study, we show that PPARgamma is upregulated during the resolution phase of wound repair concomitant with the shift to PGD2, and may be responsible for initiating endogenous mechanism resulting in healing/resolution.
Figures






Similar articles
-
Contrasting effects of peroxisome-proliferator-activated receptor (PPAR)gamma agonists on membrane-associated prostaglandin E2 synthase-1 in IL-1beta-stimulated rat chondrocytes: evidence for PPARgamma-independent inhibition by 15-deoxy-Delta12,14prostaglandin J2.Arthritis Res Ther. 2005;7(6):R1325-37. doi: 10.1186/ar1830. Epub 2005 Sep 22. Arthritis Res Ther. 2005. PMID: 16277686 Free PMC article.
-
The functional link between microsomal prostaglandin E synthase-1 (mPGES-1) and peroxisome proliferator-activated receptor γ (PPARγ) in the onset of inflammation.Pharmacol Res. 2020 Jul;157:104807. doi: 10.1016/j.phrs.2020.104807. Epub 2020 Apr 22. Pharmacol Res. 2020. PMID: 32330552
-
The endogenous bioactive lipid prostaglandin D2-glycerol ester reduces murine colitis via DP1 and PPARγ receptors.FASEB J. 2018 Sep;32(9):5000-5011. doi: 10.1096/fj.201701205R. Epub 2018 Apr 9. FASEB J. 2018. PMID: 29630407
-
Dual acting anti-inflammatory drugs: a reappraisal.Pharmacol Res. 2001 Dec;44(6):437-50. doi: 10.1006/phrs.2001.0872. Pharmacol Res. 2001. PMID: 11735348 Review.
-
The anti-inflammatory effects of prostaglandins.J Investig Med. 2009 Aug;57(6):703-8. doi: 10.2310/JIM.0b013e31819aaa76. J Investig Med. 2009. PMID: 19240648 Review.
Cited by
-
Vegetable omega-3 and omega-6 fatty acids differentially modulate the antiviral and antibacterial immune responses of Atlantic salmon.Sci Rep. 2024 May 13;14(1):10947. doi: 10.1038/s41598-024-61144-w. Sci Rep. 2024. PMID: 38740811 Free PMC article.
-
PPARγ downregulation by TGFß in fibroblast and impaired expression and function in systemic sclerosis: a novel mechanism for progressive fibrogenesis.PLoS One. 2010 Nov 2;5(11):e13778. doi: 10.1371/journal.pone.0013778. PLoS One. 2010. PMID: 21072170 Free PMC article.
-
mPGES-1 null mice are resistant to bleomycin-induced skin fibrosis.Arthritis Res Ther. 2011;13(1):R6. doi: 10.1186/ar3226. Epub 2011 Jan 25. Arthritis Res Ther. 2011. PMID: 21266028 Free PMC article.
-
Chemosensitivity and mechanosensitivity of nociceptors from incised rat hindpaw skin.Anesthesiology. 2009 Jul;111(1):155-64. doi: 10.1097/ALN.0b013e3181a16443. Anesthesiology. 2009. PMID: 19512876 Free PMC article.
-
Effect of the Combined Compound Probiotics with Glycyrrhinic Acid on Alleviating Cytotoxicity of IPEC-J2 Cells Induced by Multi-Mycotoxins.Toxins (Basel). 2022 Sep 27;14(10):670. doi: 10.3390/toxins14100670. Toxins (Basel). 2022. PMID: 36287939 Free PMC article.
References
-
- Kapoor M, Kojima F, Appleton I, Kawai S, Crofford LJ. Major enzymatic pathways in dermal wound healing: current understanding and future therapeutic targets. Curr Opin Investig Drugs. 2006;7:418–422. - PubMed
-
- Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999:698–701. - PubMed
-
- Ianaro A, Ialenti A, Maffia P, Pisano B, Di Rosa M. Role of cyclopentenone prostaglandins in rat carrageenin pleurisy. FEBS Lett. 2001;508:61–66. - PubMed
-
- Kapoor M, Clarkson AN, Sutherland BA, Appleton I. The role of antioxidants in models of inflammation: emphasis on L-arginine and arachidonic acid metabolism. Inflammopharmacology. 2005;12:505–519. - PubMed
-
- Crofford LJ. Prostaglandin biology. Gastroenterol Clin North Am. 2001;30:863–876. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

