Nestin-GFP reporter expression defines the quiescent state of skeletal muscle satellite cells
- PMID: 17239845
- PMCID: PMC1888564
- DOI: 10.1016/j.ydbio.2006.12.026
Nestin-GFP reporter expression defines the quiescent state of skeletal muscle satellite cells
Abstract
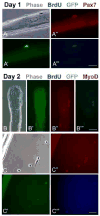
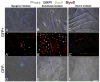
Repair of adult skeletal muscle depends on satellite cells, quiescent myogenic stem cells located beneath the myofiber basal lamina. Satellite cell numbers and performance decline with age and disease, yet the intrinsic molecular changes accompanying these conditions are unknown. We identified expression of GFP driven by regulatory elements of the nestin (NES) gene within mouse satellite cells, which permitted characterization of these cells in their niche. Sorted NES-GFP+ cells exclusively acquired a myogenic fate, even when supplemented with media supporting non-myogenic development. Mutual and unique gene expression by NES-GFP+ cells from hindlimb and diaphragm muscles demonstrated intra- and inter-muscular heterogeneity of satellite cells. NES-GFP expression declined following satellite cell activation and was reacquired in late stage myogenic cultures by non-proliferating Pax7+ progeny. The dynamics of this expression pattern reflect the cycle of satellite cell self-renewal. The NES-GFP model reveals unique transcriptional activity within quiescent satellite cells and permits novel insight into the heterogeneity of their molecular signatures.
Figures





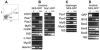

References
-
- Aihara M, Sugawara K, Torii S, Hosaka M, Kurihara H, Saito N, Takeuchi T. Angiogenic endothelium-specific nestin expression is enhanced by the first intron of the nestin gene. Lab Invest. 2004;84:1581–1592. - PubMed
-
- Banwell BL. Intermediate filament-related myopathies. Pediatr Neurol. 2001;24:257–263. - PubMed
-
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

