Sulfonation of 17beta-estradiol and inhibition of sulfotransferase activity by polychlorobiphenylols and celecoxib in channel catfish, Ictalurus punctatus
- PMID: 17239972
- PMCID: PMC1993540
- DOI: 10.1016/j.aquatox.2006.12.011
Sulfonation of 17beta-estradiol and inhibition of sulfotransferase activity by polychlorobiphenylols and celecoxib in channel catfish, Ictalurus punctatus
Abstract
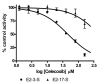
The sulfonation of 17beta-estradiol (E2) by human liver and recombinant sulfotransferases is influenced by environmental contaminants such as hydroxylated metabolites of polychlorinated biphenyls (OH-PCBs), which are potent inhibitors, and the therapeutic drug, celecoxib, which affects positional sulfonation of E2. In some locations, the aquatic environment is contaminated by PCBs, OH-PCBs and widely used therapeutic drugs. The objectives of this study were to investigate the sulfonation kinetics of E2 in liver cytosol from channel catfish (Ictalurus punctatus); to examine the effect of OH-PCBs on E2 sulfonation; and to determine if celecoxib altered the position of E2 sulfonation, as it does with human liver cytosol. E2 was converted to both 3- and 17-sulfates by catfish liver cytosol. At E2 concentrations below 1 microM, formation of E2-3-sulfate (E2-3-S) predominated, but substrate inhibition was observed at higher concentrations. Rates of E2-3-S formation at different E2 concentrations were fit to a substrate inhibition model, with K'm and V'max values of 0.40 +/- 0.10 microM and 91.0 +/- 4.7 pmol/min/mg protein, respectively and K(i) of 1.08 +/- 0.09 microM. The formation of E2-17-S fit Michaelis-Menten kinetics over the concentration range 25 nM to 2.5 microM, with K(m) and V(max) values of 1.07 +/- 0.23 microM and 25.7 +/- 4.43 pmol/min/mg protein, respectively. The efficiency (V(max)/K(m)) of formation of E2-3-S was 9.8-fold higher than that of E2-17-S. Several OH-PCBs inhibited E2 3-sulfonation, measured at an E2 concentration of 1 nM. Of those tested, the most potent inhibitor was 4'-OH-CB79, with two chlorine atoms flanking the OH group (IC(50): 94 nM). The inhibition of estrogen sulfonation by OH-PCBs may disrupt the endocrine system and thus contribute to the known toxic effects of these compounds. Celecoxib did not stimulate E2-17-S formation, as is the case with human liver cytosol, but did inhibit the formation of E2-3-S (IC(50): 44 microM) and to a lesser extent, E2-17-S (IC(50): > 160 microM), suggesting the previously found effect of celecoxib on E2-17-S formation may be specific to human SULT2A1.
Figures


Similar articles
-
Sulfotransferase 2A1 forms estradiol-17-sulfate and celecoxib switches the dominant product from estradiol-3-sulfate to estradiol-17-sulfate.J Steroid Biochem Mol Biol. 2005 Sep;96(5):367-74. doi: 10.1016/j.jsbmb.2005.05.002. Epub 2005 Jul 11. J Steroid Biochem Mol Biol. 2005. PMID: 16011896
-
Celecoxib affects estrogen sulfonation catalyzed by several human hepatic sulfotransferases, but does not stimulate 17-sulfonation in rat liver.J Steroid Biochem Mol Biol. 2017 Sep;172:46-54. doi: 10.1016/j.jsbmb.2017.05.012. Epub 2017 May 25. J Steroid Biochem Mol Biol. 2017. PMID: 28552400 Free PMC article.
-
Polychlorobiphenylols are selective inhibitors of human phenol sulfotransferase 1A1 with 4-nitrophenol as a substrate.Chem Biol Interact. 2006 Feb 25;159(3):235-46. doi: 10.1016/j.cbi.2005.12.004. Chem Biol Interact. 2006. PMID: 16413005
-
Inhibition of sulfotransferases by xenobiotics.Curr Drug Metab. 2006 Jan;7(1):83-104. doi: 10.2174/138920006774832596. Curr Drug Metab. 2006. PMID: 16454694 Review.
-
Interactions of cytosolic sulfotransferases with xenobiotics.Drug Metab Rev. 2013 Nov;45(4):401-14. doi: 10.3109/03602532.2013.835613. Drug Metab Rev. 2013. PMID: 24188364 Review.
Cited by
-
Triclosan is a potent inhibitor of estradiol and estrone sulfonation in sheep placenta.Environ Int. 2010 Nov;36(8):942-9. doi: 10.1016/j.envint.2009.02.004. Epub 2009 Mar 18. Environ Int. 2010. PMID: 19299018 Free PMC article.
-
Sulfate metabolites of 4-monochlorobiphenyl in whole poplar plants.Environ Sci Technol. 2013 Jan 2;47(1):557-62. doi: 10.1021/es303807f. Epub 2012 Dec 14. Environ Sci Technol. 2013. PMID: 23215248 Free PMC article.
-
Influence of Tetrabromobisphenol A, with or without Concurrent Triclosan, upon Bisphenol A and Estradiol Concentrations in Mice.Environ Health Perspect. 2017 Aug 21;125(8):087014. doi: 10.1289/EHP1329. Environ Health Perspect. 2017. PMID: 28886593 Free PMC article.
-
Hydroxylated and sulfated metabolites of commonly occurring airborne polychlorinated biphenyls inhibit human steroid sulfotransferases SULT1E1 and SULT2A1.Environ Toxicol Pharmacol. 2018 Mar;58:196-201. doi: 10.1016/j.etap.2018.01.010. Epub 2018 Feb 3. Environ Toxicol Pharmacol. 2018. PMID: 29408762 Free PMC article.
References
-
- Bensen WG. Anti-inflammatory and analgesic efficacy of COX-2 specific inhibition: from investigational trials to clinical experience. J Rheumatol Suppl. 2000;60:17–24. - PubMed
-
- Chetrite GS, Cortes-Prieto J, Philippe JC, Wright F, Pasqualini JR. Comparison of estrogen concentrations, E1 sulfatase and aromatase activities in normal, and in cancerous, human breast tissues. J Steroid Biochem Mol Biol. 2000;72:23–27. - PubMed
-
- Cornish-Bowden A. Fundamentals of enzyme kinetics. 3. Portland Press Ltd; London: 2004.
-
- Coughtrie MWH. Sulphation catalysed by the human cytosolic sulphotransferases-chemical defence or molecular terrorism? Hum Exp Toxicol. 1996;15:547–555. - PubMed
-
- Cui D, Booth-Genthe CL, Carlini E, Carr B, Schrag ML. Heterotropic modulation of sulfotransferase 2A1 activity by celecoxib: product ratio switching of ethynylestradiol sulfation, Drug Metab. Dispos. 2004;32:1260–1264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

