Curvature effect on the structure of phospholipid bilayers
- PMID: 17241048
- PMCID: PMC2720570
- DOI: 10.1021/la062455t
Curvature effect on the structure of phospholipid bilayers
Abstract
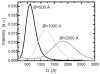
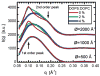
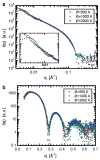
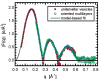
High-resolution small-angle X-ray scattering (SAXS), complemented by small-angle neutron scattering (SANS) and dynamic light scattering (DLS) experiments, was used to study the effect of curvature on the bilayer structure of dioleoyl-phosphatidylcholine (DOPC) and dioleoyl-phosphatidylserine (DOPS) unilamellar vesicles (ULVs). Bilayer curvature, as a result of finite vesicle size, was varied as a function of vesicle radius and determined by DLS and SANS measurements. Unilamellarity of large DOPC ULVs was achieved by the addition of small amounts (up to 4 mol %) of the charged lipid, DOPS. A comparison of SANS data over the range of 0.02 < q <0.2 A-1 indicated no change in the overall bilayer thickness as a function of ULV diameter (620 to 1840 A). SANS data were corroborated by high-resolution (0.06 < q <0.6 A-1) SAXS data for the same diameter ULVs and data obtained from planar samples of aligned bilayers. Both the inner and outer leaflets of the bilayer were found to be indistinguishable. This observation agrees well with simple geometric models describing the effect of vesicle curvature. However, 1220-A-diameter pure DOPS ULVs form asymmetric bilayers whose structure can most likely be rationalized in terms of geometrical constraints coupled with electrostatic interactions, rather than curvature alone.
Figures


References
-
- Balgavý P, Dubničková M, Kučerka N, Kiselev MA, Yaradaikin SP, Uhríková D. Biochim Biophys Acta. 2001;1512:40–52. - PubMed
-
- Katsaras J, Gutberlet T. Lipid Bilayers: Structure and Interactions. Springer-Verlag; New York: 2001.
-
- Krueger S, Ankner JF, Satija SK, Majkrzak CF, Gurley D, Colombini M. Langmuir. 1995;11:3218–3222.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

