The sensitivity of murine spermiogenesis to miglustat is a quantitative trait: a pharmacogenetic study
- PMID: 17241468
- PMCID: PMC1794412
- DOI: 10.1186/1477-7827-5-1
The sensitivity of murine spermiogenesis to miglustat is a quantitative trait: a pharmacogenetic study
Abstract
Background: A major event in the post-meiotic development of male germ cells is the formation of the acrosome. This process can be perturbed in C57BL/6 mice by administration of the small molecule miglustat (N-butyldeoxynojirimycin, NB-DNJ). The miglustat-treated mice produce morphologically abnormal spermatozoa that lack acrosomes and are poorly motile. In C57BL/6 mice, miglustat can be used to maintain long-term reversible infertility. In contrast, when miglustat was evaluated in normal men, it did not affect spermatogenesis. To gain more insight into this species difference we have now evaluated the reproductive effects of miglustat in rabbits, in multiple mouse strains and in interstrain hybrid mice.
Methods: Male mice of 18 inbred strains were administered miglustat orally or via miniosmotic pumps. Rabbits were given the compound in their food. Fourth-generation interstrain hybrid mice, bred from C57BL/6 and FVB/N mice (which differ in their response to miglustat), also received the drug. Data on fertility (natural mating), sperm motility and morphology, acrosome status, and serum drug levels were collected.
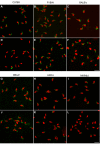
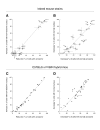
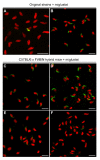
Results: In rabbits the drug did not induce aberrations of sperm shape or motility, although the serum level of miglustat in rabbits far exceeded the level in C57BL/6 mice (8.4 microM and 0.5 microM, respectively). In some strains of the Swiss and Castle lineages of inbred mice miglustat did not cause infertility, severe morphological sperm aberrations or reduced sperm motility. In these strains miglustat only had milder effects. However, miglustat strongly disturbed acrosome and sperm nucleus development in AKR/J and BALB/c mice and in a number of C57BL/6-related strains. The consequences of drug administration in the interstrain hybrid mice were highly variable. Judging by the number of grossly abnormal spermatozoa, these genetically heterogeneous mice displayed a continuous range of intermediate responses, distinct from either of their parental strains.
Conclusion: The effects of miglustat on spermatogenesis in mice are strain-dependent, while in rabbits the drug is ineffective. Evaluation of interstrain hybrid mice indicated that the sensitivity of spermatogenesis to miglustat is a quantitative trait. These studies pave the way for identifying the genetic factors underlying the strain/species differences in the effect of miglustat.
Figures



Similar articles
-
Miglustat has no apparent effect on spermatogenesis in normal men.Hum Reprod. 2007 Mar;22(3):702-7. doi: 10.1093/humrep/del414. Epub 2006 Oct 25. Hum Reprod. 2007. PMID: 17067996
-
Differential sensitivity of mouse strains to an N-alkylated imino sugar: glycosphingolipid metabolism and acrosome formation.Pharmacogenomics. 2008 Jun;9(6):717-31. doi: 10.2217/14622416.9.6.717. Pharmacogenomics. 2008. PMID: 18518850 Free PMC article. Review.
-
Alkylated imino sugars, reversible male infertility-inducing agents, do not affect the genetic integrity of male mouse germ cells during short-term treatment despite induction of sperm deformities.Biol Reprod. 2005 Apr;72(4):805-13. doi: 10.1095/biolreprod.104.036053. Epub 2004 Dec 1. Biol Reprod. 2005. PMID: 15576825
-
Reversible infertility in male mice after oral administration of alkylated imino sugars: a nonhormonal approach to male contraception.Proc Natl Acad Sci U S A. 2002 Dec 24;99(26):17173-8. doi: 10.1073/pnas.262586099. Epub 2002 Dec 11. Proc Natl Acad Sci U S A. 2002. PMID: 12477936 Free PMC article.
-
Haploid male germ cells-the Grand Central Station of protein transport.Hum Reprod Update. 2020 Jun 18;26(4):474-500. doi: 10.1093/humupd/dmaa004. Hum Reprod Update. 2020. PMID: 32318721 Review.
Cited by
-
Species-specific differences in nonlysosomal glucosylceramidase GBA2 function underlie locomotor dysfunction arising from loss-of-function mutations.J Biol Chem. 2019 Mar 15;294(11):3853-3871. doi: 10.1074/jbc.RA118.006311. Epub 2019 Jan 20. J Biol Chem. 2019. PMID: 30662006 Free PMC article.
-
Synthesis and evaluation of eight- and four-membered iminosugar analogues as inhibitors of testicular ceramide-specific glucosyltransferase, testicular β-glucosidase 2, and other glycosidases.J Org Chem. 2012 Apr 6;77(7):3082-98. doi: 10.1021/jo202054g. Epub 2012 Mar 20. J Org Chem. 2012. PMID: 22432895 Free PMC article.
-
Truncated mutants of beta-glucosidase 2 (GBA2) are localized in the mitochondrial matrix and cause mitochondrial fragmentation.PLoS One. 2020 Jun 3;15(6):e0233856. doi: 10.1371/journal.pone.0233856. eCollection 2020. PLoS One. 2020. PMID: 32492073 Free PMC article.
-
Loss of function of glucocerebrosidase GBA2 is responsible for motor neuron defects in hereditary spastic paraplegia.Am J Hum Genet. 2013 Feb 7;92(2):238-44. doi: 10.1016/j.ajhg.2012.11.021. Epub 2013 Jan 17. Am J Hum Genet. 2013. PMID: 23332916 Free PMC article.
-
New therapies in the management of Niemann-Pick type C disease: clinical utility of miglustat.Ther Clin Risk Manag. 2009;5:877-87. doi: 10.2147/tcrm.s5777. Epub 2009 Nov 18. Ther Clin Risk Manag. 2009. PMID: 19956552 Free PMC article.
References
-
- Jeyakumar M, Dwek RA, Butters TD, Platt FM. Storage solutions: treating lysosomal disorders of the brain. Nat Rev Neurosci. 2005;6:713–725. - PubMed
-
- Cox T, Lachmann R, Hollak C, Aerts J, van Weely S, Hrebicek M, Platt F, Butters T, Dwek R, Moyses C, Gow I, Elstein D, Zimran A. Novel oral treatment of Gaucher's disease with N-butyldeoxynojirimycin (OGT 918) to decrease substrate biosynthesis. Lancet. 2000;355:1481–1485. doi: 10.1016/S0140-6736(00)02161-9. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

