Antibody-based enrichment of peptides on magnetic beads for mass-spectrometry-based quantification of serum biomarkers
- PMID: 17241609
- PMCID: PMC1852426
- DOI: 10.1016/j.ab.2006.12.023
Antibody-based enrichment of peptides on magnetic beads for mass-spectrometry-based quantification of serum biomarkers
Abstract
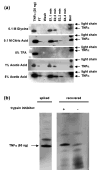
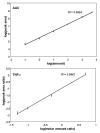
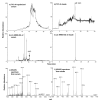
A major bottleneck for validation of new clinical diagnostics is the development of highly sensitive and specific assays for quantifying proteins. We previously described a method, stable isotope standards with capture by antipeptide antibodies, wherein a specific tryptic peptide is selected as a stoichiometric representative of the protein from which it is cleaved, is enriched from biological samples using immobilized antibodies, and is quantitated using mass spectrometry against a spiked internal standard to yield a measure of protein concentration. In this study, we optimized a magnetic-bead-based platform amenable to high-throughput peptide capture and demonstrated that antibody capture followed by mass spectrometry can achieve ion signal enhancements on the order of 10(3), with precision (CVs <10%) and accuracy (relative error approximately 20%) sufficient for quantifying biomarkers in the physiologically relevant ng/mL range. These methods are generally applicable to any protein or biological fluid of interest and hold great potential for providing a desperately needed bridging technology between biomarker discovery and clinical application.
Figures


References
-
- Clemons M, Danson S, Howell A. Tamoxifen (“Nolvadex”): a review. Cancer Treat Rev. 2002;28:165–80. - PubMed
-
- Haber DA, Bell DW, Sordella R, Kwak EL, Godin-Heymann N, Sharma SV, Lynch TJ, Settleman J. Molecular Targeted Therapy of Lung Cancer: EGFR Mutations and Response to EGFR Inhibitors. Cold Spring Harb Symp Quant Biol. 2005;70:419–26. - PubMed
-
- Ligibel JA, Winer EP. Trastuzumab/chemotherapy combinations in metastatic breast cancer. Semin Oncol. 2002;29:38–43. - PubMed
-
- Shawver LK, Slamon D, Ullrich A. Smart drugs: tyrosine kinase inhibitors in cancer therapy. Cancer Cell. 2002;1:117–23. - PubMed
-
- Fitzgibbons PL, Page DL, Weaver D, Thor AD, Allred DC, Clark GM, Ruby SG, O’Malley F, Simpson JF, Connolly JL, Hayes DF, Edge SB, Lichter A, Schnitt SJ. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:966–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

