Effects of UMB24 and (+/-)-SM 21, putative sigma2-preferring antagonists, on behavioral toxic and stimulant effects of cocaine in mice
- PMID: 17241657
- PMCID: PMC1892169
- DOI: 10.1016/j.pbb.2006.12.011
Effects of UMB24 and (+/-)-SM 21, putative sigma2-preferring antagonists, on behavioral toxic and stimulant effects of cocaine in mice
Abstract
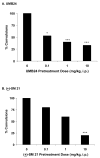
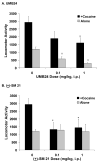
Earlier studies have demonstrated that antagonism of sigma1 receptors attenuates the convulsive, lethal, locomotor stimulatory and rewarding actions of cocaine in mice. In contrast, the contribution of sigma2 receptors is unclear because experimental tools to selectively target this subtype are unavailable. To begin addressing this need, we characterized UMB24 (1-(2-phenethyl)-4-(2-pyridyl)-piperazine) and (+/-)-SM 21 (3alpha-tropanyl-2-(4-chorophenoxy)butyrate) in receptor binding and behavioral studies. Receptor binding studies confirmed that UMB24 and (+/-)-SM 21 display preferential affinity for sigma2 over sigma1 receptors. In behavioral studies, pretreatment of Swiss Webster mice with UMB24 or (+/-)-SM 21 significantly attenuated cocaine-induced convulsions and locomotor activity, but not lethality. When administered alone, (+/-)-SM 21 produced no significant effects compared to control injections of saline, but UMB24 had locomotor depressant actions. Together, the data suggest that sigma2 receptor antagonists have the potential to attenuate some of the behavioral effects of cocaine, and further development of more selective, high affinity ligands are warranted.
Figures
Similar articles
-
(+/-)-SM 21 attenuates the convulsive and locomotor stimulatory effects of cocaine in mice.Eur J Pharmacol. 2001 Apr 6;417(1-2):R1-2. doi: 10.1016/s0014-2999(01)00891-3. Eur J Pharmacol. 2001. PMID: 11301071
-
Involvement of sigma (sigma) receptors in the acute actions of methamphetamine: receptor binding and behavioral studies.Neuropharmacology. 2005 Oct;49(5):638-45. doi: 10.1016/j.neuropharm.2005.04.016. Neuropharmacology. 2005. PMID: 15939443
-
Interactions between 3,4-methylenedioxymethamphetamine and sigma1 receptors.Eur J Pharmacol. 2006 Dec 28;553(1-3):141-5. doi: 10.1016/j.ejphar.2006.09.038. Epub 2006 Sep 28. Eur J Pharmacol. 2006. PMID: 17070798 Free PMC article.
-
Involvement of the sigma1 receptor in the appetitive effects of cocaine.Pharmacopsychiatry. 2004 Nov;37 Suppl 3:S198-207. doi: 10.1055/s-2004-832678. Pharmacopsychiatry. 2004. PMID: 15547786 Review.
-
Sigma receptors: potential medications development target for anti-cocaine agents.Eur J Pharmacol. 2003 May 23;469(1-3):1-12. doi: 10.1016/s0014-2999(03)01723-0. Eur J Pharmacol. 2003. PMID: 12782179 Review.
Cited by
-
Cocaine Effects on Dopaminergic Transmission Depend on a Balance between Sigma-1 and Sigma-2 Receptor Expression.Front Mol Neurosci. 2018 Feb 12;11:17. doi: 10.3389/fnmol.2018.00017. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29483862 Free PMC article.
-
The Sigma-2 receptor / transmembrane protein 97 (σ2R/TMEM97) modulator JVW-1034 reduces heavy alcohol drinking and associated pain states in male mice.Neuropharmacology. 2021 Feb 15;184:108409. doi: 10.1016/j.neuropharm.2020.108409. Epub 2020 Nov 20. Neuropharmacology. 2021. PMID: 33221481 Free PMC article.
-
Sigma1 receptor antagonists determine the behavioral pattern of the methamphetamine-induced stereotypy in mice.Psychopharmacology (Berl). 2009 May;203(4):781-92. doi: 10.1007/s00213-008-1425-z. Epub 2008 Dec 4. Psychopharmacology (Berl). 2009. PMID: 19052726 Free PMC article.
-
Synthesis and σ1 Receptor Binding of Halogenated N,N'-Diphenethylethylenediamines.Med Chem (Los Angeles). 2011 Dec 25;1(102):1000102. doi: 10.4172/2161-0444.1000102. Med Chem (Los Angeles). 2011. PMID: 23565348 Free PMC article.
-
Pharmacological Analysis of the Anti-epileptic Mechanisms of Fenfluramine in scn1a Mutant Zebrafish.Front Pharmacol. 2017 Apr 6;8:191. doi: 10.3389/fphar.2017.00191. eCollection 2017. Front Pharmacol. 2017. PMID: 28428755 Free PMC article.
References
-
- Bertha CM, Vilner BJ, Mattson MV, Bowen WD, Becketts K, Xu H, Rothman RB, Flippen-Anderson JL, Rice KC. (E)-8-Benzylidene derivatives of 2-methyl-5-(3-hydroxyphenyl)morphans: highly selective ligands for the sigma 2 receptor subtype. J Med Chem. 1995;38:4776–85. - PubMed
-
- Boja JW, Kuhar MJ, Kopajtic T, Yang E, Abraham P, Lewin AH, et al. Secondary amine analogues of 3β-(4′-substituted phenyl)tropane-2β-carboxylic acid esters and N-norcocaine exhibit enhanced affinity for serotonin and norepinephrine transporters. J Med Chem. 1994;37:1220–3. - PubMed
-
- Bouchard P, Quirion R. [3H]1,3-Di(2-tolyl)guanidine and [3H](±)-pentazocine binding sites in the rat brain: autoradiographic visualization of the putative sigma1 and sigma2 receptor subtypes. Neuroscience. 1997;6:467–77. - PubMed
-
- Bowen WD, Bertha CM, Vilner BJ, Rice KC. CB-64D and CB-184: Ligands with high σ2 receptor affinity and subtype selectivity. Eur J Pharmacol. 1995a;278:257–60. - PubMed
-
- Bowen WD, Vilner BJ, Williams W, Bertha CM, Kuehne ME, Jacobson AE. Ibogaine and its congeners are sigma 2 receptor-selective ligands with moderate affinity. Eur J Pharmacol. 1995b;279:R1–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

