Palmitoylation-dependent protein sorting
- PMID: 17242068
- PMCID: PMC2063950
- DOI: 10.1083/jcb.200610151
Palmitoylation-dependent protein sorting
Abstract
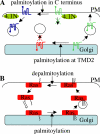
S-palmitoylation is a posttranslational modification that regulates membrane-protein interactions. However, palmitate is more than just a hydrophobic membrane anchor, as many different types of protein are palmitoylated, including transmembrane proteins. Indeed, there is now compelling evidence that palmitoylation plays a key role in regulating various aspects of protein sorting within the cell.
Figures

References
-
- Ali, M.R., K.H. Cheng, and J. Huang. 2006. Ceramide drives cholesterol out of the ordered lipid bilayer phase into the crystal phase in 1-palmitoyl- 2-oleoyl-glycero-3-phosphocholine/cholesterol/ceramide ternary mixtures. Biochemistry. 45:12629–12638. - PubMed
-
- Baker, T.L., H. Zheng, J. Walker, J.L. Coloff, and J.E. Buss. 2003. Distinct rates of palmitate turnover on membrane-bound cellular and oncogenic H-Ras. J. Biol. Chem. 278:19292–19300. - PubMed
-
- Choy, E., V.K. Chiu, J. Silletti, M. Feoktistov, T. Morimoto, D. Michaelson, I.E. Ivanov, and M.R. Philips. 1999. Endomembrane trafficking of Ras: The CAAX motif targets proteins to the ER and Golgi. Cell. 98:69–80. - PubMed
-
- Craven, S.E., A.E. El-Husseini, and D.S. Bredt. 1999. Synaptic targeting of the postsynaptic density protein PSD-95 mediated by lipid and protein motifs. Neuron. 22:497–509. - PubMed
-
- de Planque, M.R.R., and J.A. Killian. 2003. Protein-lipid interactions studied with designed transmembrane peptides: role of hydrophobic matching and interfacial anchoring. Mol. Membr. Biol. 20:271–284. - PubMed

