Viral induction of AID is independent of the interferon and the Toll-like receptor signaling pathways but requires NF-kappaB
- PMID: 17242162
- PMCID: PMC2118730
- DOI: 10.1084/jem.20061801
Viral induction of AID is independent of the interferon and the Toll-like receptor signaling pathways but requires NF-kappaB
Abstract
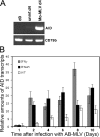
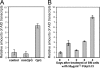
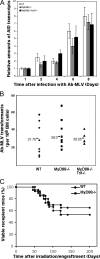
Activation-induced cytidine deaminase (AID) is expressed in germinal centers of lymphoid organs during immunoglobulin diversification, in bone marrow B cells after infection with Abelson murine leukemia retrovirus (Ab-MLV), and in human B cells after infection by hepatitis C virus. To understand how viruses signal AID induction in the host we asked whether the AID response was abrogated in cells deficient in the interferon pathway or in signaling via the Toll-like receptors. Here we show that AID is not an interferon responsive gene and abrogation of Toll-like receptor signaling does not diminish the AID response. However, we found that NF-kappaB was required for expression of virally induced AID. Since NF-kappaB binds and activates the AID promoter, these results mechanistically link viral infection with AID transcription. Thus, induction of AID by viruses could be the result of several signaling pathways that culminate in NF-kappaB activation, underscoring the versatility of this host defense program.
Figures


Similar articles
-
Expression of activation-induced cytidine deaminase in human hepatocytes via NF-kappaB signaling.Oncogene. 2007 Aug 16;26(38):5587-95. doi: 10.1038/sj.onc.1210344. Epub 2007 Apr 2. Oncogene. 2007. PMID: 17404578
-
IL-6 production is positively regulated by two distinct Src homology domain 2-containing tyrosine phosphatase-1 (SHP-1)-dependent CCAAT/enhancer-binding protein β and NF-κB pathways and an SHP-1-independent NF-κB pathway in lipopolysaccharide-stimulated bone marrow-derived macrophages.J Immunol. 2011 May 1;186(9):5443-56. doi: 10.4049/jimmunol.1003551. Epub 2011 Mar 21. J Immunol. 2011. PMID: 21422245
-
Regulation of the MIR155 host gene in physiological and pathological processes.Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
-
B cell receptor induced Fc receptor-like 5 expression is mediated by multiple signaling pathways converging on NF-κB and NFAT.Mol Immunol. 2016 May;73:112-21. doi: 10.1016/j.molimm.2016.04.001. Epub 2016 Apr 8. Mol Immunol. 2016. PMID: 27065451
-
Back to homeostasis: Negative regulation of NF-κB immune signaling in insects.Dev Comp Immunol. 2018 Oct;87:216-223. doi: 10.1016/j.dci.2018.06.007. Epub 2018 Jun 14. Dev Comp Immunol. 2018. PMID: 29908201 Review.
Cited by
-
AID expression increased by TNF-α is associated with class switch recombination of Igα gene in cancers.Cell Mol Immunol. 2016 Jul;13(4):484-91. doi: 10.1038/cmi.2015.26. Epub 2015 Apr 6. Cell Mol Immunol. 2016. PMID: 25849121 Free PMC article.
-
Regulation of AID expression in the immune response.J Exp Med. 2007 May 14;204(5):1145-56. doi: 10.1084/jem.20061952. Epub 2007 Apr 23. J Exp Med. 2007. PMID: 17452520 Free PMC article.
-
Expression of infectious murine leukemia viruses by RAW264.7 cells, a potential complication for studies with a widely used mouse macrophage cell line.Retrovirology. 2008 Jan 4;5:1. doi: 10.1186/1742-4690-5-1. Retrovirology. 2008. PMID: 18177500 Free PMC article.
-
Activation of the NF-kappaB pathway by adeno-associated virus (AAV) vectors and its implications in immune response and gene therapy.Proc Natl Acad Sci U S A. 2011 Mar 1;108(9):3743-8. doi: 10.1073/pnas.1012753108. Epub 2011 Feb 14. Proc Natl Acad Sci U S A. 2011. Retraction in: Proc Natl Acad Sci U S A. 2018 Jan 9;115(2):E343. doi: 10.1073/pnas.1720655115. PMID: 21321191 Free PMC article. Retracted.
-
TRAF2 Deficiency in B Cells Impairs CD40-Induced Isotype Switching That Can Be Rescued by Restoring NF-κB1 Activation.J Immunol. 2018 Dec 1;201(11):3421-3430. doi: 10.4049/jimmunol.1800337. Epub 2018 Oct 19. J Immunol. 2018. PMID: 30341187 Free PMC article.
References
-
- Ramiro, A.R., M. Jankovic, T. Eisenreich, S. Difilippantonio, S. Chen-Kiang, M. Muramatsu, T. Honjo, A. Nussenzweig, and M.C. Nussenzweig. 2004. AID is required for c-myc/IgH chromosome translocations in vivo. Cell. 118:431–438. - PubMed
-
- Sayegh, C.E., M.W. Quong, Y. Agata, and C. Murre. 2003. E-proteins directly regulate expression of activation-induced deaminase in mature B cells. Nat. Immunol. 4:586–593. - PubMed
-
- Dedeoglu, F., B. Horwitz, J. Chaudhuri, F.W. Alt, and R.S. Geha. 2004. Induction of activation-induced cytidine deaminase gene expression by IL-4 and CD40 ligation is dependent on STAT6 and NFkappaB. Int. Immunol. 16:395–404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

