TAL-1/SCL and its partners E47 and LMO2 up-regulate VE-cadherin expression in endothelial cells
- PMID: 17242194
- PMCID: PMC1899886
- DOI: 10.1128/MCB.00493-06
TAL-1/SCL and its partners E47 and LMO2 up-regulate VE-cadherin expression in endothelial cells
Abstract
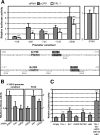
The basic helix-loop-helix TAL-1/SCL essential for hematopoietic development is also required during vascular development for embryonic angiogenesis. We reported that TAL-1 acts positively on postnatal angiogenesis by stimulating endothelial morphogenesis. Here, we investigated the functional consequences of TAL-1 silencing in human primary endothelial cells. We found that TAL-1 knockdown caused the inhibition of in vitro tubulomorphogenesis, which was associated with a dramatic reduction in vascular endothelial cadherin (VE-cadherin) at intercellular junctions. Consistently, silencing of TAL-1 as well as of its cofactors E47 and LMO2 down-regulated VE-cadherin at both the mRNA and the protein level. Endogenous VE-cadherin transcription could be activated in nonendothelial HEK-293 cells by the sole concomitant ectopic expression of TAL-1, E47, and LMO2. Transient transfections in human primary endothelial cells derived from umbilical vein (HUVECs) demonstrated that VE-cadherin promoter activity was dependent on the integrity of a specialized E-box associated with a GATA motif and was maximal with the coexpression of the different components of the TAL-1 complex. Finally, chromatin immunoprecipitation assays showed that TAL-1 and its cofactors occupied the VE-cadherin promoter in HUVECs. Together, these data identify VE-cadherin as a bona fide target gene of the TAL-1 complex in the endothelial lineage, providing a first clue to TAL-1 function in angiogenesis.
Figures




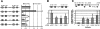

References
-
- Batlle, E., E. Sancho, C. Franci, D. Dominguez, M. Monfar, J. Baulida, and A. Garcia De Herreros. 2000. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2:84-89. - PubMed
-
- Carmeliet, P., M. G. Lampugnani, L. Moons, F. Breviario, V. Compernolle, F. Bono, G. Balconi, R. Spagnuolo, B. Oostuyse, M. Dewerchin, A. Zanetti, A. Angellilo, V. Mattot, D. Nuyens, E. Lutgens, F. Clotman, M. C. de Ruiter, A. Gittenberger-de Groot, R. Poelmann, F. Lupu, J. M. Herbert, D. Collen, and E. Dejana. 1999. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 98:147-157. - PubMed
-
- Chetty, R., M. A. Dada, C. H. Boshoff, M. A. Comley, S. C. Biddolph, J. W. Schneider, D. Y. Mason, K. A. Pulford, and K. C. Gatter. 1997. TAL-1 protein expression in vascular lesions. J. Pathol. 181:311-315. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
