The 2'-O-methyltransferase responsible for modification of yeast tRNA at position 4
- PMID: 17242307
- PMCID: PMC1800514
- DOI: 10.1261/rna.399607
The 2'-O-methyltransferase responsible for modification of yeast tRNA at position 4
Abstract
The methylation of the ribose 2'-OH of RNA occurs widely in nature and in all stable RNAs and occurs at five positions in yeast tRNA. 2'-O-methylation of tRNA at position 4 is interesting because it occurs in the acceptor stem (which is normally undermodified), it is the only 2'-O-methylation that occurs in the middle of a duplex region in tRNA, the modification is conserved in eukaryotes, and the features of the tRNA necessary for substrate recognition are poorly defined. We show here that Saccharomyces cerevisiae ORF YOL125w (TRM13) is necessary and sufficient for 2'-O-methylation at position 4 of yeast tRNA. Biochemical analysis of the S. cerevisiae proteome shows that Trm13 copurifies with 2'-O-methylation activity, using tRNAGlyGCC as a substrate, and extracts made from a trm13-Delta strain have undetectable levels of this activity. Trm13 is necessary for activity in vivo because tRNAs isolated from a trm13-Delta strain lack the corresponding 2'-O-methylated residue for each of the three known tRNAs with this modification. Trm13 is sufficient for 2'-O-methylation at position 4 in vitro since yeast Trm13 protein purified after expression in Escherichia coli has the same activity as that produced in yeast. Trm13 protein binds substrates tRNAHis and tRNAGlyGCC with KD values of 85+/-8 and 100+/-14 nM, respectively, and has a KM for tRNAHis of 10 nM, but binds nonsubstrate tRNAs very poorly (KD>1 microM). Trm13 is conserved in eukaryotes, but there is no sequence similarity between Trm13 and other known methyltransferases.
Figures





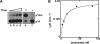

Similar articles
-
Identification of the yeast gene encoding the tRNA m1G methyltransferase responsible for modification at position 9.RNA. 2003 May;9(5):574-85. doi: 10.1261/rna.5070303. RNA. 2003. PMID: 12702816 Free PMC article.
-
Unexpected expansion of tRNA substrate recognition by the yeast m1G9 methyltransferase Trm10.RNA. 2013 Aug;19(8):1137-46. doi: 10.1261/rna.039651.113. Epub 2013 Jun 21. RNA. 2013. PMID: 23793893 Free PMC article.
-
A domain of the actin binding protein Abp140 is the yeast methyltransferase responsible for 3-methylcytidine modification in the tRNA anti-codon loop.RNA. 2011 Jun;17(6):1100-10. doi: 10.1261/rna.2652611. Epub 2011 Apr 25. RNA. 2011. PMID: 21518804 Free PMC article.
-
Two-subunit enzymes involved in eukaryotic post-transcriptional tRNA modification.RNA Biol. 2014;11(12):1608-18. doi: 10.1080/15476286.2015.1008360. RNA Biol. 2014. PMID: 25625329 Free PMC article. Review.
-
Enzymatic formation of N2,N2-dimethylguanosine in eukaryotic tRNA: importance of the tRNA architecture.Biochimie. 1995;77(1-2):54-61. doi: 10.1016/0300-9084(96)88104-1. Biochimie. 1995. PMID: 7599276 Review.
Cited by
-
Epitranscriptome: Review of Top 25 Most-Studied RNA Modifications.Int J Mol Sci. 2022 Nov 10;23(22):13851. doi: 10.3390/ijms232213851. Int J Mol Sci. 2022. PMID: 36430347 Free PMC article. Review.
-
Trm13p, the tRNA:Xm4 modification enzyme from Saccharomyces cerevisiae is a member of the Rossmann-fold MTase superfamily: prediction of structure and active site.J Mol Model. 2010 Mar;16(3):599-606. doi: 10.1007/s00894-009-0570-6. Epub 2009 Aug 22. J Mol Model. 2010. PMID: 19697067
-
2'-O-ribose methylation of transfer RNA promotes recovery from oxidative stress in Saccharomyces cerevisiae.PLoS One. 2020 Feb 13;15(2):e0229103. doi: 10.1371/journal.pone.0229103. eCollection 2020. PLoS One. 2020. PMID: 32053677 Free PMC article.
-
Charting new territory: The Plasmodium falciparum tRNA modification landscape.Biomed J. 2025 Apr;48(2):100745. doi: 10.1016/j.bj.2024.100745. Epub 2024 May 9. Biomed J. 2025. PMID: 38734409 Free PMC article. Review.
-
tRNA biology charges to the front.Genes Dev. 2010 Sep 1;24(17):1832-60. doi: 10.1101/gad.1956510. Genes Dev. 2010. PMID: 20810645 Free PMC article. Review.
References
-
- Alexandrov, A., Dutta, K., Pascal, S.M. MBP fusion protein with a viral protease cleavage site: One-step cleavage/purification of insoluble proteins. Biotechniques. 2001;30:1194–1198. - PubMed
-
- Alexandrov, A., Vignali, M., LaCount, D.J., Quartley, E., de Vries, C., De Rosa, D., Babulski, J., Mitchell, S.F., Schoenfeld, L.W., Fields, S., et al. A facile method for high-throughput co-expression of protein pairs. Mol. Cell. Proteomics. 2004;3:934–938. - PubMed
-
- Alexandrov, A., Chernyakov, I., Gu, W., Hiley, S.L., Hughes, T.R., Grayhack, E.J., Phizicky, E.M. Rapid tRNA decay can result from lack of nonessential modifications. Mol. Cell. 2006;21:87–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
