Hepatitis B virus core antigen epitopes presented by HLA-A2 single-chain trimers induce functional epitope-specific CD8+ T-cell responses in HLA-A2.1/Kb transgenic mice
- PMID: 17244158
- PMCID: PMC2265916
- DOI: 10.1111/j.1365-2567.2007.02543.x
Hepatitis B virus core antigen epitopes presented by HLA-A2 single-chain trimers induce functional epitope-specific CD8+ T-cell responses in HLA-A2.1/Kb transgenic mice
Abstract
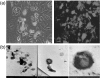
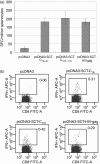
The potency of CD8+ cytotoxic T lymphocyte (CTL) responses toward core antigen has been shown to affect the outcomes of hepatitis B virus (HBV) infection. Since single-chain trimers (SCT) composed of peptide epitope beta2-microglobulin (beta2m) and major histocompatibility complex (MHC) class I heavy chain covalently linked together in a single molecule have been shown to stimulate efficient CTL responses, we investigated the properties of human leucocyte antigen (HLA)-A2 SCTs encoding the HBV core antigen (HBcAg) epitopes C(18-27) and C(107-115). Transfection of NIH-3T3 cells with pcDNA3.0-SCT-C(18-27) and SCT-C(107-115) leads to stable presentation of HBcAg epitopes at the cell surface. HLA-A2.1/Kb transgenic mice vaccinated with the SCT constructs, either as a DNA vaccine alone or followed by a boost with recombinant vaccinia virus, were shown to generate HBcAg-specific CTL responses by enzyme-linked immunospot assay (ELISPOT) and in vitro interferon-gamma release experiments. HBcAg-specific CTLs from vaccinated HLA-A2.1/Kb transgenic mice were able to inhibit HBV surface and e antigen expression as indicated by HepG2.2.15 cells. Our data indicate that a DNA vaccine encoding a human HLA-A2 SCT with HBV epitopes can lead to stable, enhanced HBV core antigen presentation, and may be useful for the control of HBV infection in HLA-A2-positive HBV carriers.
Figures




References
-
- Yang SH, Lee CG, Park SH, et al. Correlation of antiviral T-cell responses with suppression of viral rebound in chronic hepatitis B carriers: a proof-of-concept study. Gene Ther. 2006;13:1110–17. - PubMed
-
- Lohr HF, Gerken G, Schlicht HJ, Meryer zum Buschenfelde KH, Fleischer B. Low frequency of cytotoxic liver-infiltrating T lymphocytes specific for endogenous processed surface and core proteins in chronic hepatitis B. J Infect Dis. 1993;168:1133–9. - PubMed
-
- Lohr HF, Krug S, Herr W, Weyer S, Schlaak J, Wolfel T, Gerken G, Meyer zum Buschenfelde KH. Quantitative and functional analysis of core-specific T-helper cell and CTL activities in acute and chronic hepatitis B. Liver. 1998;18:405–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

