Studies on the interactions between C-reactive protein and complement proteins
- PMID: 17244159
- PMCID: PMC2265924
- DOI: 10.1111/j.1365-2567.2007.02535.x
Studies on the interactions between C-reactive protein and complement proteins
Abstract
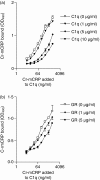
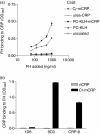
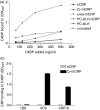
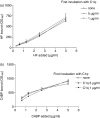
Several studies have investigated the interactions between C-reactive protein (CRP) and various complement proteins but none of them took into consideration the different structural forms of CRP. The aim of our study was to investigate whether the different antigenic forms of CRP are able to bind C1q, to trigger activation of the C1 complex and to study the ability of the various CRP forms to bind complement factor H (FH) and C4b-binding protein (C4BP). Interactions between various CRP forms and complement proteins were analysed in enzyme-linked immunosorbent assay and surface plasmon resonance tests and activation of the C1 complex was followed in a reconstituted system using purified C1q, C1r and C1s in the presence of C1-INH. Native, ligand-unbound CRP activated the classical pathway weakly. After binding to phosphocholine, native CRP bound C1q and significantly activated C1. Native CRP complexed to phosphocholine did not bind the complement regulatory proteins FH and C4BP. After disruption of the pentameric structure of CRP, as achieved by urea-treatment or by site-directed mutagenesis, C1q binding and C1 activation further increased and the ability of CRP to bind complement regulatory proteins was revealed. C1q binds to CRP through its globular head domain. The binding sites on CRP for FH and C4BP seemed to be different from that of C1q. In conclusion, in parallel with the increase in the C1-activating ability of different CRP structural variants, the affinity for complement regulatory proteins also increased, providing the biological basis for limitation of excess complement activation.
Figures




References
-
- Volanakis JE. Human C-reactive protein. Expression, structure and function. Mol Immunol. 2001;38:189–97. - PubMed
-
- Pepys MB. C-reactive protein fifty years on. Lancet. 1981;1(8221):653–7. - PubMed
-
- Du Clos TW, Mold C. C-reactive protein: an activator of innate immunity and a modulator of adaptive immunity. Immunol Res. 2004;30:261–77. - PubMed
-
- Wu Y, Wang HW, Ji SR, Sui SF. Two-dimensional crystallization of rabbit C-reactive protein monomeric subunits. Acta Crystallogr D Biol Crystallogr. 2003;59:922–6. - PubMed
-
- Kresl JJ, Potempa LA, Anderson BE. Conversion of native oligomeric to a modified monomeric form of human C-reactive protein. Int J Biochem Cell Biol. 1998;30:1415–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

