Scavenging of the cofactor lipoate is essential for the survival of the malaria parasite Plasmodium falciparum
- PMID: 17244193
- PMCID: PMC2796473
- DOI: 10.1111/j.1365-2958.2007.05592.x
Scavenging of the cofactor lipoate is essential for the survival of the malaria parasite Plasmodium falciparum
Abstract
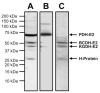
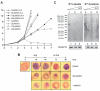
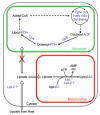
Lipoate is an essential cofactor for key enzymes of oxidative metabolism. Plasmodium falciparum possesses genes for lipoate biosynthesis and scavenging, but it is not known if these pathways are functional, nor what their relative contribution to the survival of intraerythrocytic parasites might be. We detected in parasite extracts four lipoylated proteins, one of which cross-reacted with antibodies against the E2 subunit of apicoplast-localized pyruvate dehydrogenase (PDH). Two highly divergent parasite lipoate ligase A homologues (LplA), LipL1 (previously identified as LplA) and LipL2, restored lipoate scavenging in lipoylation-deficient bacteria, indicating that Plasmodium has functional lipoate-scavenging enzymes. Accordingly, intraerythrocytic parasites scavenged radiolabelled lipoate and incorporated it into three proteins likely to be mitochondrial. Scavenged lipoate was not attached to the PDH E2 subunit, implying that lipoate scavenging drives mitochondrial lipoylation, while apicoplast lipoylation relies on biosynthesis. The lipoate analogue 8-bromo-octanoate inhibited LipL1 activity and arrested P. falciparum in vitro growth, decreasing the incorporation of radiolabelled lipoate into parasite proteins. Furthermore, growth inhibition was prevented by lipoate addition in the medium. These results are consistent with 8-bromo-octanoate specifically interfering with lipoate scavenging. Our study suggests that lipoate metabolic pathways are not redundant, and that lipoate scavenging is critical for Plasmodium intraerythrocytic survival.
Figures


References
-
- Bender A, van Dooren GG, Ralph SA, McFadden GI, Schneider G. Properties and prediction of mitochondrial transit peptides from Plasmodium falciparum. Mol Biochem Parasitol. 2003;132:59–66. - PubMed
-
- Booker SJ. Unraveling the pathway of lipoic acid biosynthesis. Chem Biol. 2004;11:10–12. - PubMed
-
- Bryk R, Lima CD, Erdjument-Bromage H, Tempst P, Nathan C. Metabolic enzymes of mycobacteria linked to antioxidant defense by a thioredoxin-like protein. Science. 2002;295:1073–1077. - PubMed
-
- Bunik VI. 2-Oxo acid dehydrogenase complexes in redox regulation. Eur J Biochem. 2003;270:1036–1042. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

