Genetic variants of CYP3A5, CYP2D6, SULT1A1, UGT2B15 and tamoxifen response in postmenopausal patients with breast cancer
- PMID: 17244352
- PMCID: PMC1851378
- DOI: 10.1186/bcr1640
Genetic variants of CYP3A5, CYP2D6, SULT1A1, UGT2B15 and tamoxifen response in postmenopausal patients with breast cancer
Abstract
Introduction: Tamoxifen therapy reduces the risk of recurrence and prolongs the survival of oestrogen-receptor-positive patients with breast cancer. Even if most patients benefit from tamoxifen, many breast tumours either fail to respond or become resistant. Because tamoxifen is extensively metabolised by polymorphic enzymes, one proposed mechanism underlying the resistance is altered metabolism. In the present study we investigated the prognostic and/or predictive value of functional polymorphisms in cytochrome P450 3A5 CYP3A5 (*3), CYP2D6 (*4), sulphotransferase 1A1 (SULT1A1; *2) and UDP-glucuronosyltransferase 2B15 (UGT2B15; *2) in tamoxifen-treated patients with breast cancer.
Methods: In all, 677 tamoxifen-treated postmenopausal patients with breast cancer, of whom 238 were randomised to either 2 or 5 years of tamoxifen, were genotyped by using PCR with restriction fragment length polymorphism or PCR with denaturing high-performance liquid chromatography.
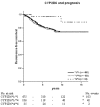
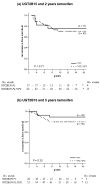
Results: The prognostic evaluation performed in the total population revealed a significantly better disease-free survival in patients homozygous for CYP2D6*4. For CYP3A5, SULT1A1 and UGT2B15 no prognostic significance was observed. In the randomised group we found that for CYP3A5, homozygous carriers of the *3 allele tended to have an increased risk of recurrence when treated for 2 years with tamoxifen, although this was not statistically significant (hazard ratio (HR) = 2.84, 95% confidence interval (CI) = 0.68 to 11.99, P = 0.15). In the group randomised to 5 years' tamoxifen the survival pattern shifted towards a significantly improved recurrence-free survival (RFS) among CYP3A5*3-homozygous patients (HR = 0.20, 95% CI = 0.07 to 0.55, P = 0.002). No reliable differences could be seen between treatment duration and the genotypes of CYP2D6, SULT1A1 or UGT2B15. The significantly improved RFS with prolonged tamoxifen treatment in CYP3A5*3 homozygotes was also seen in a multivariate Cox model (HR = 0.13, CI = 0.02 to 0.86, P = 0.03), whereas no differences could be seen for CYP2D6, SULT1A1 and UGT2B15.
Conclusion: The metabolism of tamoxifen is complex and the mechanisms responsible for the resistance are unlikely to be explained by a single polymorphism; instead it is a combination of several mechanisms. However, the present data suggest that genetic variation in CYP3A5 may predict response to tamoxifen therapy.
Figures



Similar articles
-
Association of genetic variation in tamoxifen-metabolizing enzymes with overall survival and recurrence of disease in breast cancer patients.Breast Cancer Res Treat. 2005 Jun;91(3):249-58. doi: 10.1007/s10549-004-7751-x. Breast Cancer Res Treat. 2005. PMID: 15952058
-
Genotype of metabolic enzymes and the benefit of tamoxifen in postmenopausal breast cancer patients.Breast Cancer Res. 2005;7(3):R284-90. doi: 10.1186/bcr993. Epub 2005 Jan 28. Breast Cancer Res. 2005. PMID: 15987423 Free PMC article.
-
A polymorphism in the TC21 promoter associates with an unfavorable tamoxifen treatment outcome in breast cancer.Cancer Res. 2008 Dec 1;68(23):9799-808. doi: 10.1158/0008-5472.CAN-08-0247. Cancer Res. 2008. PMID: 19047159
-
Tamoxifen, cytochrome P450 genes and breast cancer clinical outcomes.Breast. 2011 Apr;20(2):111-8. doi: 10.1016/j.breast.2010.11.003. Epub 2010 Dec 24. Breast. 2011. PMID: 21185724 Review.
-
Tamoxifen metabolism and its effect on endocrine treatment of breast cancer.Clin Adv Hematol Oncol. 2009 Mar;7(3):185-92. Clin Adv Hematol Oncol. 2009. PMID: 19398943 Review.
Cited by
-
Nonhormonal management of hot flashes for women on risk reduction therapy.J Natl Compr Canc Netw. 2010 Oct;8(10):1171-9. doi: 10.6004/jnccn.2010.0086. J Natl Compr Canc Netw. 2010. PMID: 20971841 Free PMC article.
-
Evaluating the Risk of Breast Cancer Recurrence and Metastasis After Adjuvant Tamoxifen Therapy by Integrating Polymorphisms in Cytochrome P450 Genes and Clinicopathological Characteristics.Front Oncol. 2021 Nov 19;11:738222. doi: 10.3389/fonc.2021.738222. eCollection 2021. Front Oncol. 2021. PMID: 34868931 Free PMC article.
-
New insights into the metabolism of tamoxifen and its role in the treatment and prevention of breast cancer.Steroids. 2007 Nov;72(13):829-42. doi: 10.1016/j.steroids.2007.07.009. Epub 2007 Jul 27. Steroids. 2007. PMID: 17765940 Free PMC article. Review.
-
Pharmacogenetic testing affects choice of therapy among women considering tamoxifen treatment.Genome Med. 2011 Oct 4;3(10):64. doi: 10.1186/gm280. Genome Med. 2011. PMID: 21970596 Free PMC article.
-
Genotype-guided tamoxifen dosing increases active metabolite exposure in women with reduced CYP2D6 metabolism: a multicenter study.J Clin Oncol. 2011 Aug 20;29(24):3232-9. doi: 10.1200/JCO.2010.31.4427. Epub 2011 Jul 18. J Clin Oncol. 2011. PMID: 21768473 Free PMC article.
References
-
- Stearns V, Johnson M, Rae JM, Morocho A, Novielli A, Bhargava P, Hayes DF, Desta Z, Flockhart DA. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95:1758–1764. - PubMed
-
- Johnson MD, Zuo H, Lee K-H, Trebley JP, Rae JM, Weatherman RV, Desta Z, Flockhart DA, Skaar TC. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat. 2004;85:151–159. doi: 10.1023/B:BREA.0000025406.31193.e8. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

