Effect of cyclooxygenase inhibition on cholesterol efflux proteins and atheromatous foam cell transformation in THP-1 human macrophages: a possible mechanism for increased cardiovascular risk
- PMID: 17244362
- PMCID: PMC1860062
- DOI: 10.1186/ar2109
Effect of cyclooxygenase inhibition on cholesterol efflux proteins and atheromatous foam cell transformation in THP-1 human macrophages: a possible mechanism for increased cardiovascular risk
Abstract
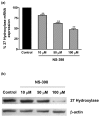
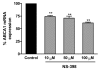
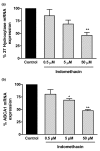
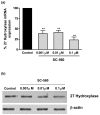
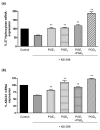
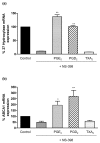
Both selective cyclooxygenase (COX)-2 inhibitors and non-steroidal anti-inflammatory drugs (NSAIDs) have been beneficial pharmacological agents for many patients suffering from arthritis pain and inflammation. However, selective COX-2 inhibitors and traditional NSAIDs are both associated with heightened risk of myocardial infarction. Possible pro-atherogenic mechanisms of these inhibitors have been suggested, including an imbalance in prostanoid production leaving the pro-aggregatory prostaglandins unopposed, but the precise mechanisms involved have not been elucidated. We explored the possibility that downregulation of proteins involved in reverse cholesterol transport away from atheromatous plaques contributes to increased atherogenesis associated with COX inhibition. The reverse cholesterol transport proteins cholesterol 27-hydroxylase and ATP-binding cassette transporter A1 (ABCA1) export cholesterol from macrophages. When mechanisms to process lipid load are inadequate, uncontrolled cholesterol deposition in macrophages transforms them into foam cells, a key element of atheromatous plaques. We showed that in cultured THP-1 human monocytes/macrophages, inhibition of COX-1, COX-2, or both reduced expression of 27-hydroxylase and ABCA1 message (real-time reverse transcription-polymerase chain reaction) and protein (immunoblot). The selective COX-2 inhibitor N-(2-cyclohexyloxy-4-nitrophenyl)methanesulfonamide (NS398) significantly reduced 27-hydroxylase and ABCA1 message (to 62.4% +/- 2.2% and 71.1% +/- 3.9% of control, respectively). Incubation with prostaglandin (PG) E2 or PGD2 reversed reductions in both of these cholesterol transport proteins induced by NS398. Cholesterol-loaded THP-1 macrophages showed significantly increased foam cell transformation in the presence of NS398 versus control (42.7% +/- 6.6% versus 20.1% +/- 3.4%, p = 0.04) as determined by oil red O staining. Pharmacological inhibition of COX in monocytes is involved in downregulation of two proteins that mediate cholesterol efflux: cholesterol 27-hydroxylase and ABCA1. Because these proteins are anti-atherogenic, their downregulation may contribute to increased incidence of cardiac events in patients treated with COX inhibitors. Reversal of inhibitory effects on 27-hydroxylase and ABCA1 expression by PGD2 and PGE2 suggests involvement of their respective signaling pathways. NS398-treated THP-1 macrophages show greater vulnerability to form foam cells. Increased cardiovascular risk with COX inhibition may be ascribed at least in part to altered cholesterol metabolism.
Figures


References
-
- Dugowson CE, Gnanashanmugam P. Nonsteroidal anti-inflammatory drugs. Phys Med Rehabil Clin N Am. 2006;17:347–354. vi. - PubMed
-
- Sanghi S, MacLaughlin EJ, Jewell CW, Chaffer S, Naus PJ, Watson LE, Dostal DE. Cyclooxygenase-2 inhibitors: a painful lesson. Cardiovasc Hematol Disord Drug Targets. 2006;6:85–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

