Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro
- PMID: 17245363
- PMCID: PMC2189767
- DOI: 10.1038/sj.bjp.0707133
Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro
Abstract
Background and purpose: A nonpsychoactive constituent of the cannabis plant, cannabidiol has been demonstrated to have low affinity for both cannabinoid CB1 and CB2 receptors. We have shown previously that cannabidiol can enhance electrically evoked contractions of the mouse vas deferens, suggestive of inverse agonism. We have also shown that cannabidiol can antagonize cannabinoid receptor agonists in this tissue with a greater potency than we would expect from its poor affinity for cannabinoid receptors. This study aimed to investigate whether these properties of cannabidiol extend to CB1 receptors expressed in mouse brain and to human CB2 receptors that have been transfected into CHO cells.
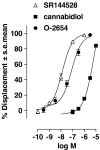
Experimental approach: The [35S]GTPS binding assay was used to determine both the efficacy of cannabidiol and the ability of cannabidiol to antagonize cannabinoid receptor agonists (CP55940 and R-(+)-WIN55212) at the mouse CB1 and the human CB2 receptor.
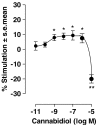
Key results: This paper reports firstly that cannabidiol displays inverse agonism at the human CB2 receptor. Secondly, we demonstrate that cannabidiol is a high potency antagonist of cannabinoid receptor agonists in mouse brain and in membranes from CHO cells transfected with human CB2 receptors.
Conclusions and implications: This study has provided the first evidence that cannabidiol can display CB2 receptor inverse agonism, an action that appears to be responsible for its antagonism of CP55940 at the human CB2 receptor. The ability of cannabidiol to behave as a CB2 receptor inverse agonist may contribute to its documented anti-inflammatory properties.
Figures
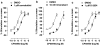

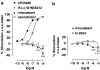
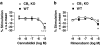
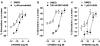
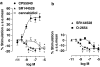


References
-
- Bouaboula M, Desnoyer N, Carayon P, Combes T, Casellas P. Gi protein modulation induced by a selective inverse agonist for the peripheral cannabinoid receptor CB2: implication for intracellular signalization cross-regulation. Mol Pharmacol. 1999;55:473–480. - PubMed
-
- Breivogel CS, Griffin G, Di Marzo V, Martin BR. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol. 2001;60:155–163. - PubMed
-
- Cheng YC, Prusoff WH. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 percent inhibition (IC50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

