Taking the time to study competitive antagonism
- PMID: 17245371
- PMCID: PMC2189774
- DOI: 10.1038/sj.bjp.0706997
Taking the time to study competitive antagonism
Abstract
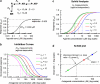
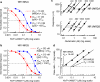
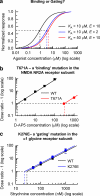
Selective receptor antagonists are one of the most powerful resources in a pharmacologist's toolkit and are essential for the identification and classification of receptor subtypes and dissecting their roles in normal and abnormal body function. However, when the actions of antagonists are measured inappropriately and misleading results are reported, confusion and wrong interpretations ensue. This article gives a general overview of Schild analysis and the method of determining antagonist equilibrium constants. We demonstrate why this technique is preferable in the study of competitive receptor antagonism than the calculation of antagonist concentration that inhibit agonist-evoked responses by 50%. In addition we show how the use of Schild analysis can provide information on the outcome of single amino acid mutations in structure-function studies of receptors. Finally, we illustrate the need for caution when studying the effects of potent antagonists on synaptic transmission where the timescale of events under investigation is such that ligands and receptors never reach steady-state occupancy.
Figures

References
-
- Banke TG, Traynelis SF. Activation of NR1/NR2B NMDA receptors. Nat Neurosci. 2003;6:144–152. - PubMed
-
- Black JW.Drugs from emasculated hormones: the principles of syntopic antagonism Noble Lectures, Physiology or Medicine 1981–1990 1993World Scientific Publishing Co.: Singapore; In: Frängsmyr T, Lindsten J (eds)
-
- Black JW, Duncan WAM, Durant CJ, Ganelin CR, Parsons EM. Definition and antagonism of histamine H2-receptors. Nature. 1972;236:385–390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

