LAMP proteins are required for fusion of lysosomes with phagosomes
- PMID: 17245426
- PMCID: PMC1783450
- DOI: 10.1038/sj.emboj.7601511
LAMP proteins are required for fusion of lysosomes with phagosomes
Abstract
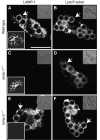
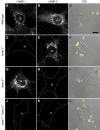
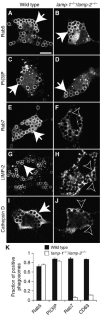
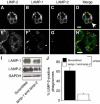
Lysosome-associated membrane proteins 1 and 2 (LAMP-1 and LAMP-2) are delivered to phagosomes during the maturation process. We used cells from LAMP-deficient mice to analyze the role of these proteins in phagosome maturation. Macrophages from LAMP-1- or LAMP-2-deficient mice displayed normal fusion of lysosomes with phagosomes. Because ablation of both the lamp-1 and lamp-2 genes yields an embryonic-lethal phenotype, we were unable to study macrophages from double knockouts. Instead, we reconstituted phagocytosis in murine embryonic fibroblasts (MEFs) by transfection of FcgammaIIA receptors. Phagosomes formed by FcgammaIIA-transfected MEFs obtained from LAMP-1- or LAMP-2- deficient mice acquired lysosomal markers. Remarkably, although FcgammaIIA-transfected MEFs from double-deficient mice ingested particles normally, phagosomal maturation was arrested. LAMP-1 and LAMP-2 double-deficient phagosomes acquired Rab5 and accumulated phosphatidylinositol 3-phosphate, but failed to recruit Rab7 and did not fuse with lysosomes. We attribute the deficiency to impaired organellar motility along microtubules. Time-lapse cinematography revealed that late endosomes/lysosomes as well as phagosomes lacking LAMP-1 and LAMP-2 had reduced ability to move toward the microtubule-organizing center, likely precluding their interaction with each other.
Figures

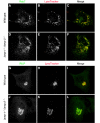
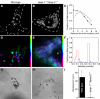
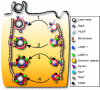
References
-
- Andrejewski N, Punnonen EL, Guhde G, Tanaka Y, Lullmann-Rauch R, Hartmann D, von Figura K, Saftig P (1999) Normal lysosomal morphology and function in LAMP-1-deficient mice. J Biol Chem 274: 12692–12701 - PubMed
-
- Blocker A, Griffiths G, Olivo JC, Hyman AA, Severin FF (1998) A role for microtubule dynamics in phagosome movement. J Cell Sci 111 (Part 3): 303–312 - PubMed
-
- Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M (1990) Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell 62: 317–329 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

