Rac1-induced cell migration requires membrane recruitment of the nuclear oncogene SET
- PMID: 17245428
- PMCID: PMC1783461
- DOI: 10.1038/sj.emboj.7601518
Rac1-induced cell migration requires membrane recruitment of the nuclear oncogene SET
Abstract
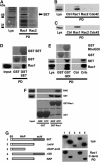
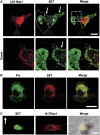
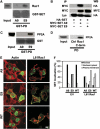
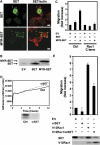
The Rho GTPase Rac1 controls cell adhesion and motility. The effector loop of Rac1 mediates interactions with downstream effectors, whereas its C-terminus binds the exchange factor beta-Pix, which mediates Rac1 targeting and activation. Here, we report that Rac1, through its C-terminus, also binds the nuclear oncogene SET/I2PP2A, an inhibitor of the serine/threonine phosphatase PP2A. We found that SET translocates to the plasma membrane in cells that express active Rac1 as well as in migrating cells. Membrane targeting of SET stimulates cell migration in a Rac1-dependent manner. Conversely, reduction of SET expression inhibits Rac1-induced migration, indicating that efficient Rac1 signalling requires membrane recruitment of SET. The recruitment of the SET oncogene to the plasma membrane represents a new feature of Rac1 signalling. Our results suggest a model in which Rac1-stimulated cell motility requires both effector loop-based downstream signalling and recruitment of a signalling amplifier, that is, SET, through the hypervariable C-terminus.
Figures

References
-
- Adachi Y, Pavlakis GN, Copeland TD (1994) Identification of in vivo phosphorylation sites of SET, a nuclear phosphoprotein encoded by the translocation breakpoint in acute undifferentiated leukemia. FEBS Lett 340: 231–235 - PubMed
-
- Anand-Apte B, Zetter BR, Viswanathan A, Qiu RG, Chen J, Ruggieri R, Symons M (1997) Platelet-derived growth factor and fibronectin-stimulated migration are differentially regulated by the Rac and extracellular signal-regulated kinase pathways. J Biol Chem 272: 30688–30692 - PubMed
-
- Berns K, Hijmans EM, Mullenders J, Brummelkamp TR, Velds A, Heimerikx M, Kerkhoven RM, Madiredjo M, Nijkamp W, Weigelt B, Agami R, Ge W, Cavet G, Linsley PS, Beijersbergen RL, Bernards R (2004) A large-scale RNAi screen in human cells identifies new components of the p53 pathway. Nature 428: 431–437 - PubMed
-
- Bos JL (2005) Linking Rap to cell adhesion. Curr Opin Cell Biol 17: 123–128 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

