Increased anxiety-like behavior and enhanced synaptic efficacy in the amygdala of GluR5 knockout mice
- PMID: 17245443
- PMCID: PMC1766473
- DOI: 10.1371/journal.pone.0000167
Increased anxiety-like behavior and enhanced synaptic efficacy in the amygdala of GluR5 knockout mice
Abstract
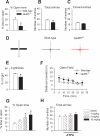
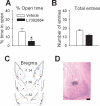
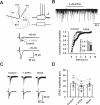
GABAergic transmission in the amygdala modulates the expression of anxiety. Understanding the interplay between GABAergic transmission and excitatory circuits in the amygdala is, therefore, critical for understanding the neurobiological basis of anxiety. Here, we used a multi-disciplinary approach to demonstrate that GluR5-containing kainate receptors regulate local inhibitory circuits, modulate the excitatory transmission from the basolateral amygdala to the central amygdala, and control behavioral anxiety. Genetic deletion of GluR5 or local injection of a GluR5 antagonist into the basolateral amygdala increases anxiety-like behavior. Activation of GluR5 selectively depolarized inhibitory neurons, thereby increasing GABA release and contributing to tonic GABA current in the basolateral amygdala. The enhanced GABAergic transmission leads to reduced excitatory inputs in the central amygdala. Our results suggest that GluR5 is a key regulator of inhibitory circuits in the amygdala and highlight the potential use of GluR5-specific drugs in the treatment of pathological anxiety.
Conflict of interest statement
Figures



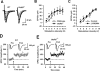
Similar articles
-
Bidirectional modulation of GABA release by presynaptic glutamate receptor 5 kainate receptors in the basolateral amygdala.J Neurosci. 2003 Jan 15;23(2):442-52. doi: 10.1523/JNEUROSCI.23-02-00442.2003. J Neurosci. 2003. PMID: 12533604 Free PMC article.
-
Activation of kainate GLU(K5) transmission rescues kindling-induced impairment of LTP in the rat lateral amygdala.Neuropsychopharmacology. 2008 Sep;33(10):2524-35. doi: 10.1038/sj.npp.1301633. Epub 2007 Nov 28. Neuropsychopharmacology. 2008. PMID: 18046310
-
Pathological alterations in GABAergic interneurons and reduced tonic inhibition in the basolateral amygdala during epileptogenesis.Neuroscience. 2009 Sep 29;163(1):415-29. doi: 10.1016/j.neuroscience.2009.06.034. Epub 2009 Jun 18. Neuroscience. 2009. PMID: 19540312 Free PMC article.
-
Mechanisms regulating GABAergic inhibitory transmission in the basolateral amygdala: implications for epilepsy and anxiety disorders.Amino Acids. 2007;32(3):305-15. doi: 10.1007/s00726-006-0415-x. Epub 2006 Oct 18. Amino Acids. 2007. PMID: 17048126 Review.
-
The basolateral amygdala γ-aminobutyric acidergic system in health and disease.J Neurosci Res. 2016 Jun;94(6):548-67. doi: 10.1002/jnr.23690. Epub 2015 Nov 19. J Neurosci Res. 2016. PMID: 26586374 Free PMC article. Review.
Cited by
-
Presynaptic facilitation of glutamate release in the basolateral amygdala: a mechanism for the anxiogenic and seizurogenic function of GluK1 receptors.Neuroscience. 2012 Sep 27;221:157-69. doi: 10.1016/j.neuroscience.2012.07.006. Epub 2012 Jul 13. Neuroscience. 2012. PMID: 22796081 Free PMC article.
-
Neurabin contributes to hippocampal long-term potentiation and contextual fear memory.PLoS One. 2008 Jan 9;3(1):e1407. doi: 10.1371/journal.pone.0001407. PLoS One. 2008. PMID: 18183288 Free PMC article.
-
Rare structural variants found in attention-deficit hyperactivity disorder are preferentially associated with neurodevelopmental genes.Mol Psychiatry. 2010 Jun;15(6):637-46. doi: 10.1038/mp.2009.57. Epub 2009 Jun 23. Mol Psychiatry. 2010. PMID: 19546859 Free PMC article.
-
Therapeutic potential of kainate receptors.CNS Neurosci Ther. 2011 Dec;17(6):661-9. doi: 10.1111/j.1755-5949.2010.00204.x. Epub 2010 Dec 6. CNS Neurosci Ther. 2011. PMID: 21129167 Free PMC article. Review.
-
Melatonin prevents sleep deprivation-associated anxiety-like behavior in rats: role of oxidative stress and balance between GABAergic and glutamatergic transmission.Am J Transl Res. 2017 May 15;9(5):2231-2242. eCollection 2017. Am J Transl Res. 2017. PMID: 28559974 Free PMC article.
References
-
- Sandford JJ, Argyropoulos SV, Nutt DJ. The psychobiology of anxiolytic drugs. Part 1: Basic neurobiology. Pharmacol Ther. 2000;88:197–212. - PubMed
-
- Rosen JB, Schulkin J. From normal fear to pathological anxiety. Psychol Rev. 1998;105:325–350. - PubMed
-
- Greenberg PE, Sisitsky T, Kessler RC, Finkelstein SN, Berndt ER, et al. The economic burden of anxiety disorders in the 1990s. J Clin Psychiatry. 1999;60:427–435. - PubMed
-
- Gross C, Hen R. The developmental origins of anxiety. Nat Rev Neurosci. 2004;5:545–552. - PubMed
-
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

